FIG 6.
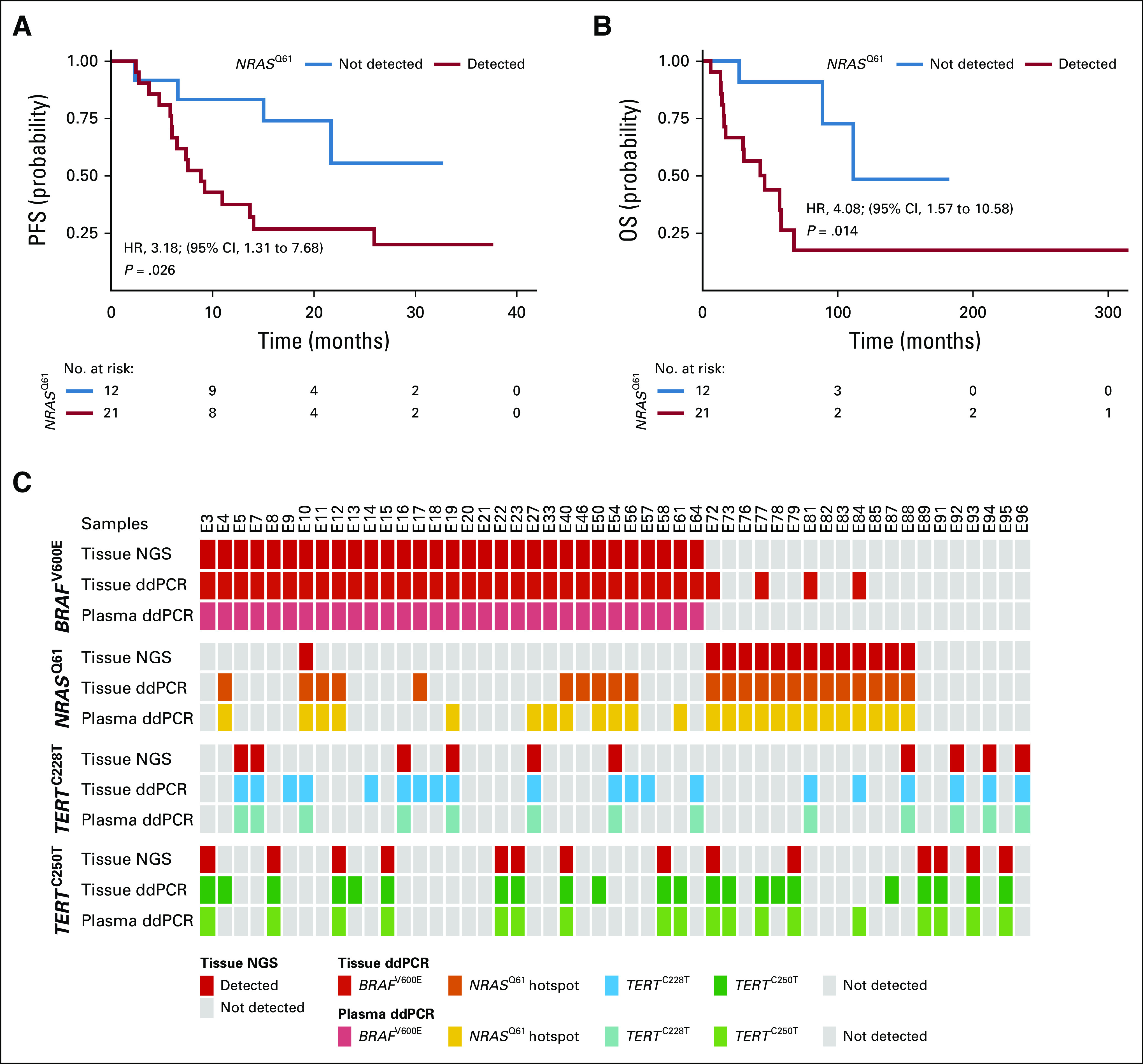
Detection of NRASQ61 at baseline (BL) is a predictor of worse clinical outcome in patients treated with mitogen-activated protein kinase (MAPK) inhibitors. Kaplan-Meier plots for (A) radiologic progression-free survival (PFS) and (B) overall survival (OS) of patients with BRAFV600E-positive tumors who received MAPK signaling targeted therapy (n = 21 with positive NRASQ61 detection in BL plasma v n = 12 without detection). Hazard ratio (HR) is reported for circulating cell-free tumor DNA (ctDNA) detected. P values were determined by the log-rank test. (C) Overview of 51 BRAFV600E-, NRASQ61-, and TERTprom-matched tumor tissue and plasma samples of patients who had available tumor biopsy specimen at therapy BL. Tumor samples were routinely analyzed for BRAFV600E, NRASQ61, and TERTprom mutations with amplicon-based next-generation sequencing (NGS; Fig. 1) and afterward reanalyzed by ddPCR. Plasma was sampled at BL (ie, before systemic therapy initiation) and analyzed for ctDNAs by droplet digital polymerase chain reaction (ddPCR).
