FIG 3.
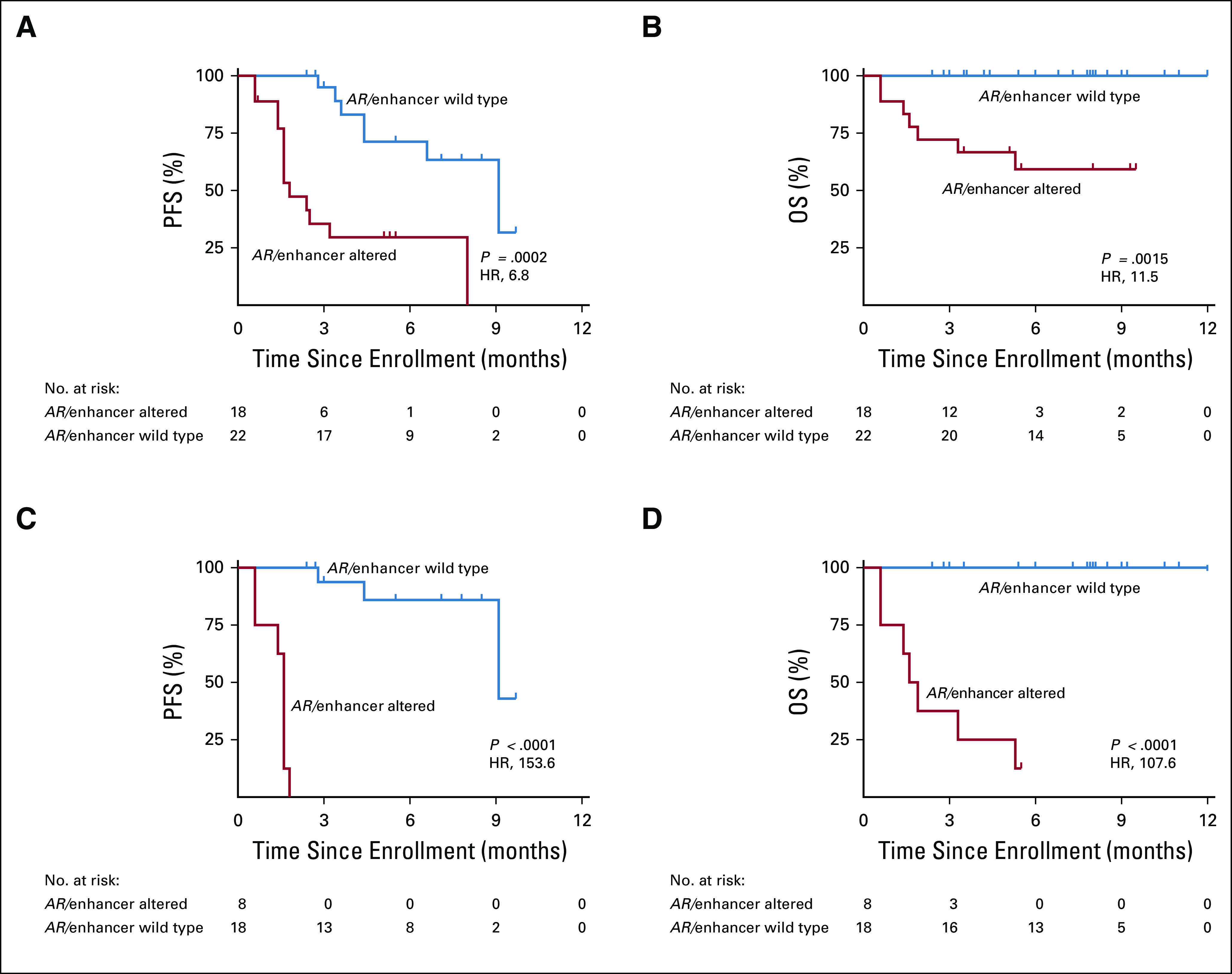
Progression-free survival (PFS) and overall survival (OS) according to androgen receptor (AR)/enhancer alteration status in cell-free DNA (cfDNA). (A) PFS, and (B) OS represent the full 40-patient cohort; (C) PFS, and (D) OS after excluding patients with secondary resistance to AR-directed therapy. Kaplan-Meier analyses were performed from the time of sample collection (time of enrollment), stratified based on the genomic alteration status of AR/enhancer measured in cfDNA. P values were calculated by the log-rank test and hazard ratios (HRs) by the Mantel-Haenszel method.
