Figure 2.
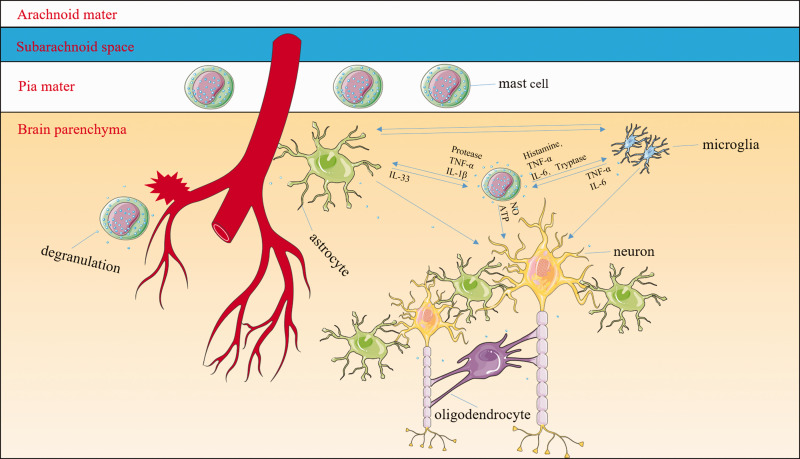
Mast cells (MCs) might be some of the central mediators of inflammatory responses. MCs are normal resident cells in the leptomeninges and brain structures surrounding blood vessels. After the onset of intracerebral hemorrhage (ICH), MCs rapidly respond by degranulating and releasing preformed and stored mediators including histamine, serotonin, heparin, TNF-α, and other cytokines. MC-derived granules can activate both microglia and astrocytes as well as exacerbate neuronal necrosis and apoptosis. For example, MC-derived tryptase induces microglial activation and secretion of pro-inflammatory factors via the protease-activated receptor 2 signaling pathway; MC-derived histamine stimulates microglial activation and the subsequent production of pro-inflammatory factors. MC proteases activate astrocytes and cause the release of IL-33 by activating p38, ERK1/2 MAPKs, and the NF-κB signaling pathway. In turn, activated microglia and astrocytes further stimulate MCs to release histamine, IL-6, and TNF-α. The interaction between MCs and oligodendrocytes is still unclear. The MC–microglia–astrocyte network amplifies the cascade of inflammatory responses and aggravates neuronal necrosis and apoptosis after ICH.