Figure 1.
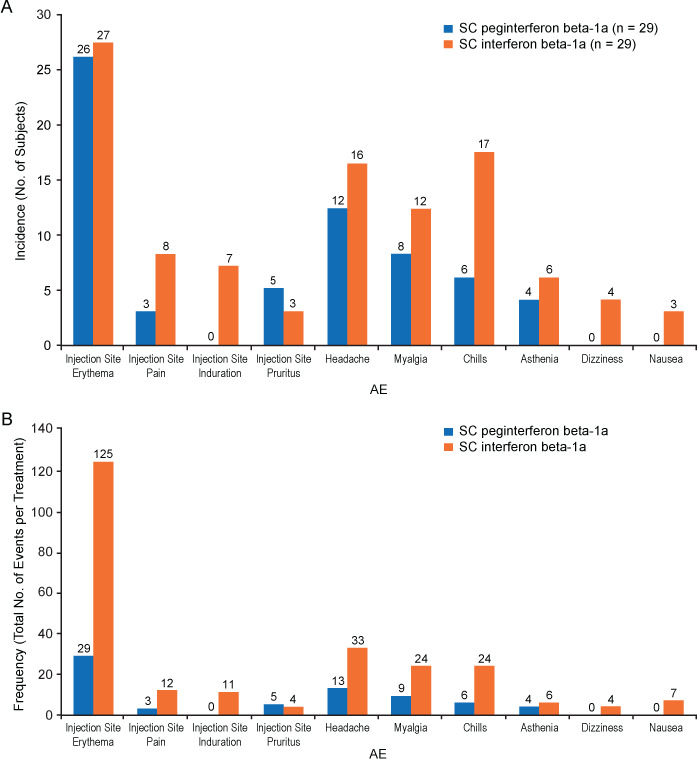
(A) Incidence and (B) frequency of adverse events (AEs) related to study drug occurring in more than 10% of participants after treatment with either peginterferon beta-1a (n = 29) or subcutaneous (SC) interferon beta-1a (n = 29) in the COMPARE study19
Reprinted with permission from Hu X, Shang S, Nestorov I, et al. COMPARE: pharmacokinetic profiles of subcutaneous peginterferon beta-1a and subcutaneous interferon beta-1a over 2 weeks in healthy subjects. Br J Clin Pharmacol. 2016;82(2):380–388. Copyright © 2016, John Wiley & Sons.
