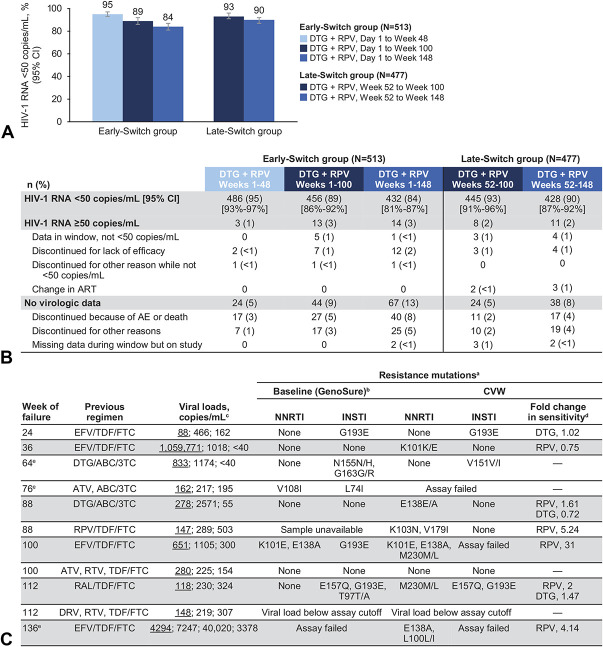
FIGURE 1.
A, Proportion of participants with plasma HIV-1 RNA <50 copies/mL at weeks 48, 100, and 148, (B) snapshot analysis at weeks 48, 100, and 148, and (C) cases of CVW through week 148. ABC, abacavir; ATV, atazanavir; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; FTC, emtricitabine; ND, not determined; NR, not reported; RAL, raltegravir; RAM, resistance-associated mutation; RPV, rilpivirine; RTV, ritonavir; 3TC, lamivudine; TDF, tenofovir disoproxil fumarate. aShading represents participants with NNRTI RAMs. bHIV-1 baseline resistance testing was performed on integrated HIV-1 proviral DNA using GenoSure Archive assay (Monogram Biosciences). On-study resistance testing used standard plasma-based genotypic and phenotypic resistance testing. cViral load values are shown; underlined value denotes viral load meeting SVW criterion followed by viral load at the confirmatory (CVW) and withdrawal visits. dFold change data were generated from assays performed at CVW in participants with observed RAMs. eParticipants in the late-switch group.