Figure 4.
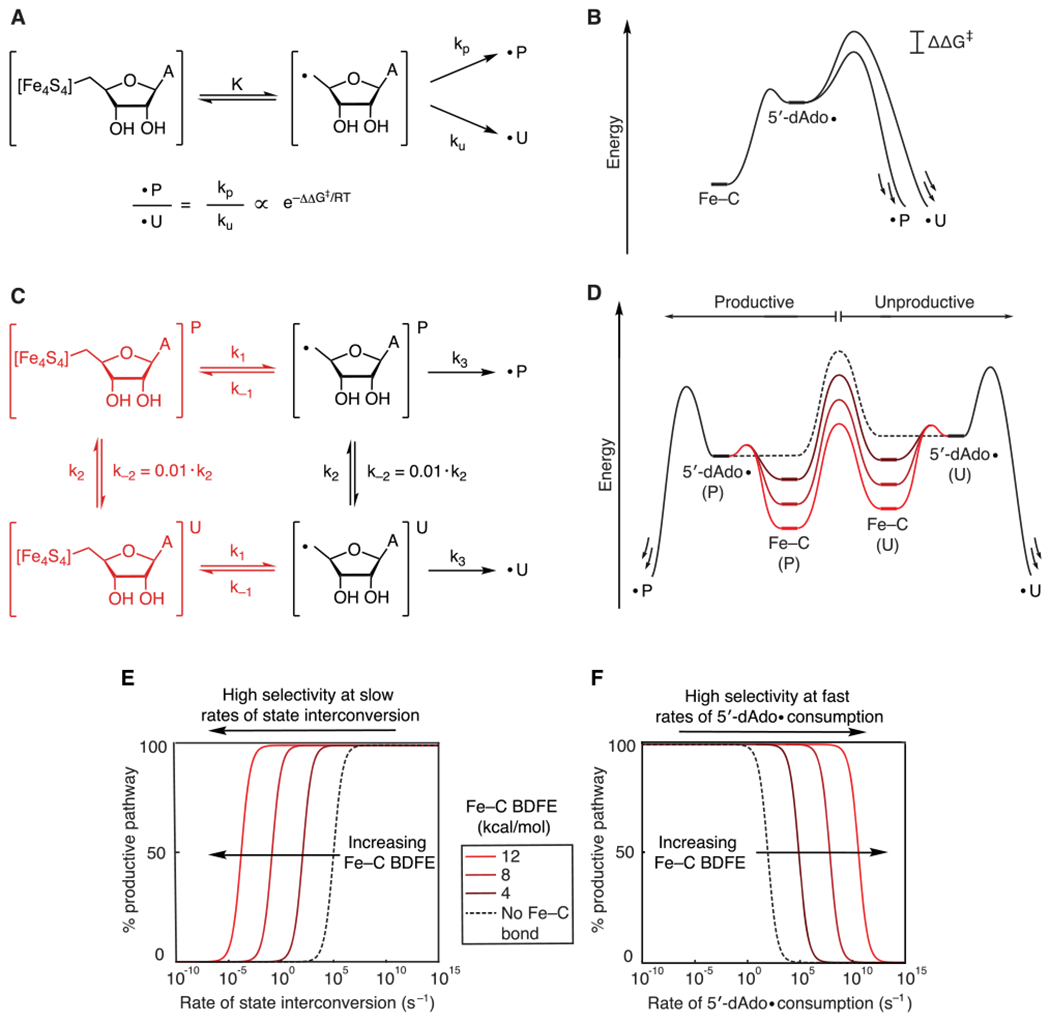
(A) Kinetic model and (B) energy diagram for a system in which the 5′-dAdo• reacts through a branching path with fixed rates of X–H abstraction. The presence of the organometallic intermediate has no effect on the selectivity. (C) Kinetic model that invokes interconversion between productive (P) and unproductive (U) states in the absence (black) and presence (black and red) of an organometallic intermediate. (D) Quantitative energy diagram for the system depicted in C showing the effect of the organometallic intermediate using barriers calculated from the rate constants listed below. As the Fe–C bond strength increases, the barrier to state interconversion becomes lower than the barrier to homolysis and X–H bond activation. (E) Simulations showing the selectivity of the reaction as a function of the rate of state interconversion and Fe–C bond strength. (F) Simulations showing the selectivity of the reaction as a function of the rate of X–H bond activation and Fe–C bond strength. k−1 (1011 s−1), k2 (102 s−2), and k3 (105 s−2) were held constant, and k2 was varied to give the indicated Fe–C bond strengths.
