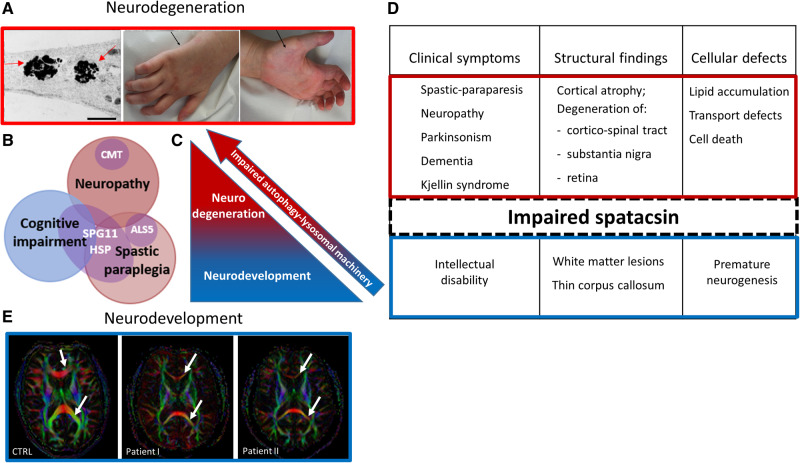
Figure 2.
Spatacsin as a mediator of the two distinct stages of SPG11-HSP pathogenesis. (A) Representation of neurodegenerative pathology in SPG11-HSP patients. Membranous inclusions (indicated by the arrows) are accumulated in the neurites of induced pluripotent stem cell-derived cortical neurons [left; adapted from Pozner et al. (2018)]. Distal neuropathy in SPG11-HSP characterized by amyotrophy of intrinsic hand muscles (arrows) and by claw hands (middle and right). (B) Heterogeneity and clinical overlap of SPG11-spectrum disorders. (C and D) Schematic representation of spatacsin-linked pathology. According to the proposed mechanism, impaired spatacsin leads to the failure of autophagy-lysosomal machinery, causing neurodevelopmental defects (blue) and over time, resulting in neurodegeneration (red). (E) Representation of neurodevelopmental pathology in SPG11-HSP patients. High-field diffusion tensor imaging (DTI) with calculation of axial fractional anisotropy maps demonstrating the thin corpus callosum as the major phenotypic hallmark of SPG11-HSP compared to controls (3 T MRI). Upper and lower arrows indicate genu and splenum of the corpus callosum, respectively, which are of significantly smaller diameter in SPG11-HSP. Scale bar = 1 μm.