Abstract
Posterior reversible encephalopathy syndrome (PRES) is a clinical syndrome that can include headache, altered consciousness, visual disturbances, and seizures, usually related to autoregulatory cerebral failure and hypertension. The neuroimaging is essential to diagnosis, showing white matter vasogenic edema in posterior areas. We present a case of a 66-year-old woman with severe pneumonia by SARS-CoV-2 who developed a posterior reversible encephalopathy syndrome with a typical clinical and radiological presentation, after being treated with anti-interleukin treatment (anakinra and tocilizumab) following local guidelines. We report a case of posterior reversible encephalopathy syndrome in a patient with COVID-19 disease, possibly related to anti-IL-1 or anti-IL-6, suggesting that anti-interleukin treatments may cause this syndrome, at least in patients with predisposing conditions such as infections and hydroelectrolytic disorders.
Keywords: Posterior reversible encephalopathy syndrome (PRES), COVID-19, Anakinra, Tocilizumab, Immunomodulators
Introduction
A 66-year-old woman with COVID-19 presented with adult respiratory distress syndrome (ARDS). Besides bilateral pneumonia, she developed multiple complications such as cardiorespiratory arrest, bacterial superinfection, hyponatremia, massive hemoptysis requiring embolization, and acute renal injury. She was started on lopinavir/ritonavir, hydroxychloroquine, and azithromycin. After radiological pulmonary progression, anti-IL-1 (daily anakinra) and anti-IL-6 (single dose of tocilizumab) were started, following local and hospital guidelines. These drugs are recommended in COVID-19 when there is clinical, blood test, or radiological progression, to avoid an excessive immunological systemic response to the virus, which is thought to worsen pulmonary infiltrates and disease prognosis. Ten days after the initiation of immunodepressants, she developed altered mental status without fever, previous headache, or visual disturbances.
Case Presentation
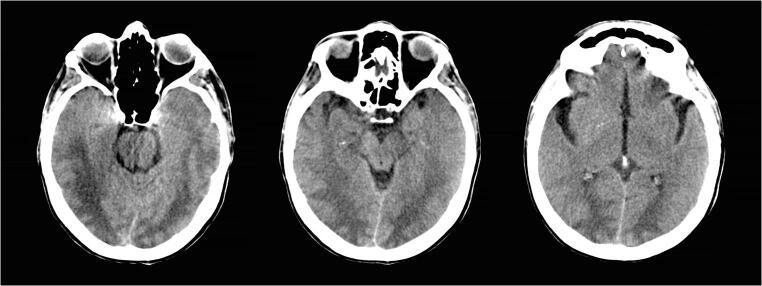
At the examination, the patient opened eyes to painful stimuli, had no verbal response, and showed withdrawal response to pain (Glasgow Coma Scale 7). The blood tests showed stable hyponatremia (130 mEq/L) and leukocytosis without any other significant findings. Her vitals were within normal limits, and blood pressure had been mildly increased during the previous 12 h with a maximum systolic pressure of 160 mmHg. Electrocardiogram showed sinus rhythm and had not atrioventricular node blocks. A CT scan with angiography was performed. There was no large vessel occlusion, no perfusion alterations, and the baseline CT (Fig. 1) showed temporo-occipital white matter hypodensity with symmetric obliteration of the sulci in that region.
Fig. 1.
CT baseline scan showed temporo-occipital symmetric white matter hypodensity
Considering the infectious background, the immunomodulatory treatments, modest hypertension in the hours before the symptoms, and the distribution of the lesions on the CT scan, the most likely diagnosis is posterior reversible encephalopathy syndrome (PRES) [1–5]. Hypertension plays a vital role in the disease due to a failure in cerebral blood flow autoregulation, and in this case, rapid rise or fluctuations in blood pressure from baseline may have been harmful, despite not being severely high [2]. The electroencephalogram showed focal slowing and epileptiform discharges in both occipital areas and ruled out nonconvulsive status epilepticus. The symptoms improved over the following days after tight control of blood pressure with labetalol infusion and discontinuing anakinra.
After 1 week, radiological infiltrates worsened and a blood test showed increased acute phase reactants. In this context, a diffuse alveolar hemorrhage was diagnosed by bronchoalveolar lavage, with suspicion of hemophagocytic syndrome. She required red blood cell transfusions and immunosupressants were restarted, as well as mechanical ventilation. The CT pulmonary scan showed worsening of infiltrates and presence of an intrapulmonary hematoma. Finally, the patient had a torpid evolution to multiorgan failure and death.
Conclusions
The absence of fever and radiological findings did not suggest an encephalitic cause of the symptoms. The serum sodium levels were only slightly decreased and had been stable for the previous days without intravenous infusion of sodium. Thus, hyponatremia was not assumed to be the cause of the sudden loss of consciousness. The cardiorespiratory arrest happened a month before the event while intubated, and it was secondary to a mucus plug after a period of desaturation and bronchospasm. It lasted less than 5 min and ended as the mucus plug was removed by fibrobronchoscopy. The post-anoxic cerebral damage was prevented by treating fever; avoiding systemic hypotension, hypoxemia, or glycemic disbalance; and continuing renal replacement therapy with hemodiafiltration instead of hemodialysis to prevent large changes in volemia. One week later, sedation was stopped and a tracheostomy was placed, and the patient progressively awakened up to a normal state of consciousness without focal neurologic signs. The timeline and posterior complete recovery from the respiratory arrest cannot explain the current episode as hypoxic-ischemic encephalopathy.
PRES has been associated with immunosuppressive and cytotoxic therapies such as platinum-containing drugs, (R)-CHOP regimens, gemcitabine, cyclosporine, tacrolimus, sirolimus, and interferon therapies. Also, agents that target angiogenesis such as bevacizumab (anti-VEGF) and tyrosine kinase inhibitors (TKI) against VEGF receptor (pazopanib, sorafenib, sunitinib) have been described as risk factors [3, 4]. Prior exposure to the predisposing drug does not appear to be protective, and patients can develop PRES even after several months after exposure [2]. Furthermore, the disorder has been associated with both acute and chronic renal disease, as was the case in our patient, and medical conditions such as hyponatremia or pulmonary infection could exacerbate the neurological findings.
Despite not being described yet, the occurrence of PRES a few days after anti-interleukin (IL-6 or IL-1) treatments which were given in this patient, raises the possibility that these kinds of immunomodulatory agents may also favor PRES.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval and Informed Consent
Consent for publication was obtained from the next of kin (daughter). Approval from the Hospital’s IRB was provided for this study.
Footnotes
This article is part of the Topical Collection on COVID-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Laura Llansó, Email: LLANSO@clinic.cat.
Xabi Urra, Email: XURRA@clinic.cat.
References
- 1.Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85:427–432. doi: 10.4065/mcp.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao B, Lyu C, Lerner A, McKinney AM. Controversy of posterior reversible encephalopathy syndrome: what have we learnt in the last 20 years? J Neurol Neurosurg Psychiatry. 2018;89:14–20. doi: 10.1136/jnnp-2017-316225. [DOI] [PubMed] [Google Scholar]
- 3.Allen JA, Adlakha A, Bergethon PR. Reversible posterior leukoencephalopathy syndrome after bevacizumab/FOLFIRI regimen for metastatic colon cancer. Arch Neurol. 2006;63:1475–1478. doi: 10.1001/archneur.63.10.1475. [DOI] [PubMed] [Google Scholar]
- 4.Ito Y, Arahata Y, Goto Y, et al. Cisplatin neurotoxicity presenting as reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol. 1998;19:415. [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz RB, Jones KM, Kalina P, Bajakian RL, Mantello MT, Garada B, Holman BL. Hypertensive encephalopathy: findings on CT, MR imaging, and SPECT imaging in 14 cases. AJR Am J Roentgenol. 1992;159:379–383. doi: 10.2214/ajr.159.2.1632361. [DOI] [PubMed] [Google Scholar]