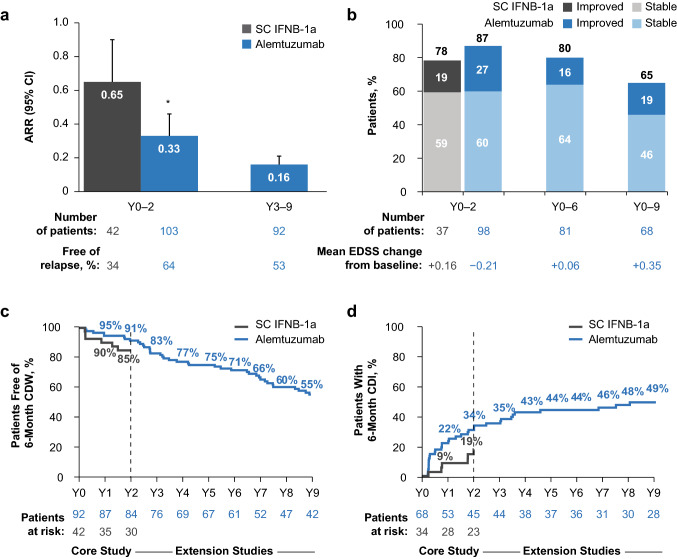
Fig. 3.
Clinical efficacy in CARE-MS II alemtuzumab-treated HAD patients through year 9: a ARR; b percentage of patients with improved or stable EDSS scores over time; may not sum appropriately due to rounding; c percentage of patients free of 6-month CDW; d percentage of patients achieving 6-month CDI. *p = 0.004 vs. SC IFNB-1a over years 0–2. Primary HAD definition: ≥ 2 relapses in the year prior to baseline and ≥ 1 Gd-enhancing lesion at baseline. Improved EDSS score: ≥ 1.0-point decrease from core study baseline; stable EDSS score: ≤ 0.5-point change in either direction from core study baseline. CDW: ≥ 1.0-point EDSS increase (or ≥ 1.5 points if baseline EDSS = 0) confirmed over 6 months. CDI: ≥ 1.0-point EDSS decrease from baseline confirmed over 6 months (assessed only in patients with baseline EDSS score ≥ 2.0). ARR annualized relapse rate, CARE-MS Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis, CDI confirmed disability improvement, CDW confirmed disability worsening, CI confidence interval, EDSS Expanded Disability Status Scale, Gd gadolinium, HAD highly active disease, SC IFNB-1a subcutaneous interferon-β-1a, Y year