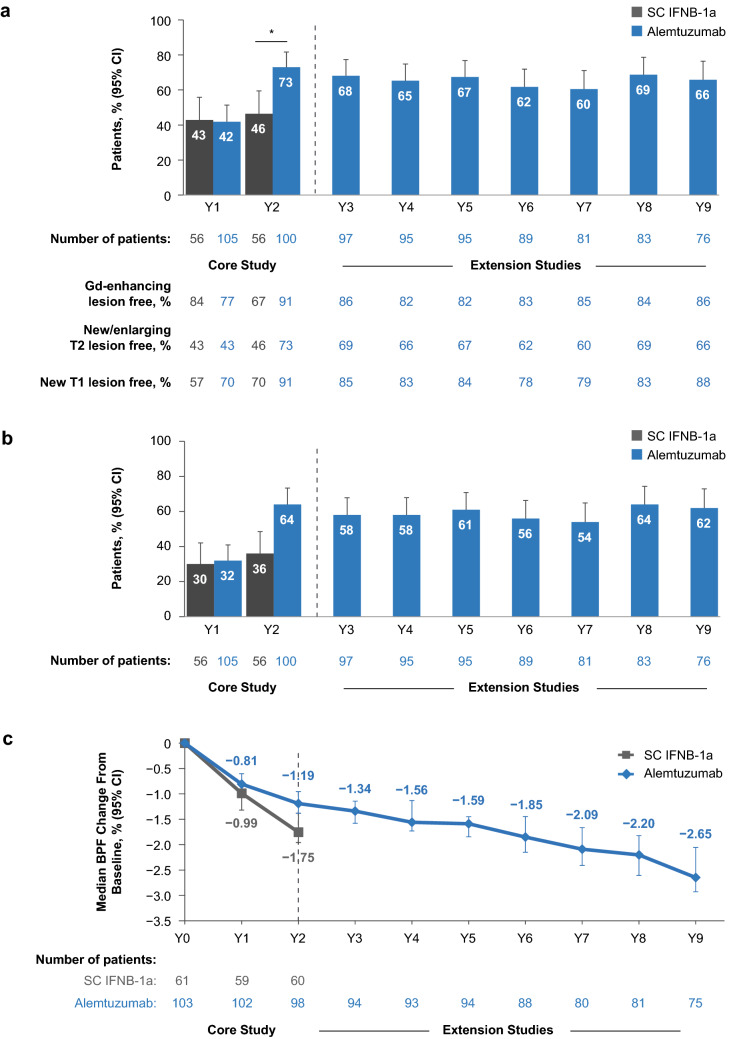
Fig. 4.
MRI outcomes and NEDA in CARE-MS I alemtuzumab-treated HAD patients through year 9: a percentage of patients free of MRI disease activity; b percentage of patients achieving annual NEDA; c cumulative BVL, change from baseline in median BPF over time. *p = 0.0007 vs. SC IFNB-1a in year 2. Primary HAD definition: ≥ 2 relapses in the year prior to baseline and ≥ 1 Gd-enhancing lesion at baseline. Freedom from MRI disease activity: no new Gd-enhancing T1 lesions on current MRI and no new/enlarging T2 hyperintense lesions since last MRI. NEDA: absence of relapse, 6-month CDW, and MRI disease activity. BPF brain parenchymal fraction, BVL brain volume loss, CARE-MS Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis, CDW confirmed disability worsening, CI confidence interval, Gd gadolinium, HAD highly active disease, MRI magnetic resonance imaging, NEDA no evidence of disease activity, SC IFNB-1a subcutaneous interferon-β-1a, Y year