Abstract
This study aimed to investigate the effects of different microwave radiation power and treatment time on the antioxidant enzyme activities and radical scavenging potency in Tartary buckwheat sprouts. The results indicated that the optimal microwave irradiation conditions for superoxide dismutase, catalase, peroxidise and ascorbate peroxidise antioxidant enzymes was the power 300 W for 75 s, and their activities were all higher than those of the control and the ungerminated seeds. In addition, under the above microwave conditions, the total reducing power and the ability to scavenge DPPH, ABTS, O2– and •OH were also optimal. These results indicated that suitable microwave treatment could effectively improve the antioxidant enzyme activity in Tartary buckwheat sprouts and enhance the antioxidant capacity of sprouts.
Keywords: Microwave, Tartary buckwheat, Antioxidant enzyme activity, Free radical scavenging rate
Introduction
Tartary buckwheat (Fagopyrum tataricum) was rich in flavonoids and polyphenolic bioactive compounds (Park et al. 2019) and used as an auxiliary materials in health food. Due to the efficient scavenging of free radicals and reduction of oxidative damage (Ma et al. 2019), it have attracted wide attention (Chavan et al. 2013).
During the growth of Tartary buckwheat sprouts, the synthesis pathway of flavonoid polyphenols is influenced by various internal and external factors, such as light, ultraviolet radiation and salt stress (Thwe et al. 2014). In adversity, plants can repair the damage caused by producing a large number of secondary metabolites, such as flavonoids and gamma-aminobutyric acid (GABA) under stress conditions. Some studies have shown that physical factors such as microwave radiation (Wang et al. 2018), magnetic fields (Zhou et al. 2012) and electric fields can induce certain effects on plant tissues (Kouchebagh et al. 2015), thereby regulating the growth of germination and embryos. Aladjadjiyan (2010) confirmed that microwaves could promote seed germination and improve the content of bioactive components. Under microwave radiation, the plant can absorb electromagnetic energy and change the structure of cell macromolecules, affecting their physiological and biochemical characteristics (Kouchebagh et al. 2015). Under adverse conditions, to control the level of ROS (reactive oxygen species) and protect cells, plant tissues can produce a variety of enzymes that scavenge ROS (Blokhina et al. 2003), such as APX, SOD, CAT, POD and other antioxidant enzymes. Under the synergistic action of these enzymes, free radicals and oxidation intermediates generated by plant metabolism can be rapidly eliminated (Zheng et al. 2018a, b). However, high power and long microwave pretreatment time can inactivate enzymes in seeds (Zhou et al. 2016) and remove the ability of gene expression (Aniszewska and Słowiński 2016). After microwave-treated seeds, the content of rutin in the sprouts increased exponentially, and the antioxidant activity was significantly improved (Nam et al. 2015). It has been reported that appropriate microwave radiation can enhance the ability of eliminating free radicals of seedlings for wheat (Chen et al. 2009) and buckwheat (Zheng et al. 2018a, b), and could significantly enhance the activity of SOD, POD, CAT, APX, GSH-Px and other antioxidant enzymes in wheat seeds (Qiu et al. 2013).
At present, the effects on the activity and scavenging ability of SOD, POD, CAT and other antioxidant enzymes in sprouts have hardly been studied (Ma et al. 2010). Therefore, this report explored the effects of microwave power and treatment time on the in vitro antioxidant enzyme activity and antioxidant capacity of Tartary buckwheat sprouts.
Materials and methods
Materials and reagents
Tartary buckwheat seeds were purchased from Ningxia Yanchi Seed Company. Chemical reagents such as 100% ethanol and other reagents were purchased from China Pharmaceutical Chemical Reagents Co., Ltd. The microwave source was a specially improved wind-powered suspension microwave oven (power: 150 − 1250 W, frequency: 2450 kHz).
Methods for Tartary buckwheat seed pretreatment
Tartary buckwheat grains were screened with a 12-mesh screen to remove debris and dirt. Full seeds were selected and rinsed with running water. A 1 g·L−1 potassium permanganate solution was used to soak the seeds at room temperature for sterilization for approximately 5 min, and the seeds were washed with distilled water. Before germination, the seeds were immersed in 25 °C distilled water for 4 h. The water was changed halfway through to facilitate seed germination with full water absorption.
Microwave treatment methods
The seeds of Tartary buckwheat were germinated after pretreatment. Seeds (3.0 g) were weighed in each batch, divided into three groups (50 seeds in each group, approximately 1 g) and evenly spread in 9 cm diameter Petri dishes. The three groups of petri dishes were uniformly placed in the microwave oven for microwave irradiation as follows. Through preliminary experiments, the following 8 treatments were determined as the research conditions: CK (no microwave treatment), 200 W for 75 s, 250 W for 75 s, 300 W for 75 s, 350 W for 75 s, 300 W for 75 s, 300 W for 30 s, 300 W for 75 s and 300 W for 120 s. The above seeds were irradiated by microwave irradiation and cultivated in a germination box (85% RH, 25 ± 2 °C) for 7 days. The seeds were washed with 8 ml distilled water every 12 h during cultivation. During this period, the germination sprouts on days 3, 5 and 7 were gathered for analysis, some of which were stored at −80 °C until use, and others were immediately freeze-dried, ground and then passed through a 40-mesh sieve prior to use.
Determination of the antioxidant enzyme activity of Tartary buckwheat
Extraction of crude enzyme solution
Samples were washed and placed in a precooled mortar, and then 50 mmol·L−1 precooled sodium phosphate buffer (2 − 3 mL) with a pH of 7.8 (containing 1% PVP and 0.1% 2-mercaptoethanol) and a small amount of quartz sand were added and ground into a homogenate in an ice bath. The supernatant was centrifuged in a centrifuge tube (4 °C, 12,000 × g, 20 min). The supernatant was the crude enzyme solution of SOD, CAT and POD. The extraction of the APX crude enzyme solution was different from that of the above mentioned buffer fluid, which was replaced by potassium phosphate buffer at pH 7.0. All steps of extracting the enzyme were carried out in a 4 °C ice bath.
Enzyme activity determination method
The SOD and CAT activity were determined according to Fikret et al. (2013). The POD and APX activity were determined according to Zhou et al. (2011).
Determination of the antioxidant activity of Tartary buckwheat sprouts
The total reducing ability was determined by the FRAP method (Chen et al. 2013). The DPPH radical scavenging ability (DPPH RSA) was determined by referencing the method of Ji et al. (2016). The ABTS radical scavenging ability (ABTS RSA) was determined by following the method of Siddhuraju and Becker (2007). The scavenging ability of the superoxide anion radical (O2–) and hydroxyl radical (•OH) was determined by referring to the method of Li et al. (2016).
Statistical analysis
Each test was repeated 3 times independently, and the results were expressed as the mean and standard deviation of the parallel measurements. SPSS software was used for one-way ANOVA and Duncan’s multiple test analysis, and the significance level was P < 0.05.
Results and discussion
APX, POD, CAT and SOD activity
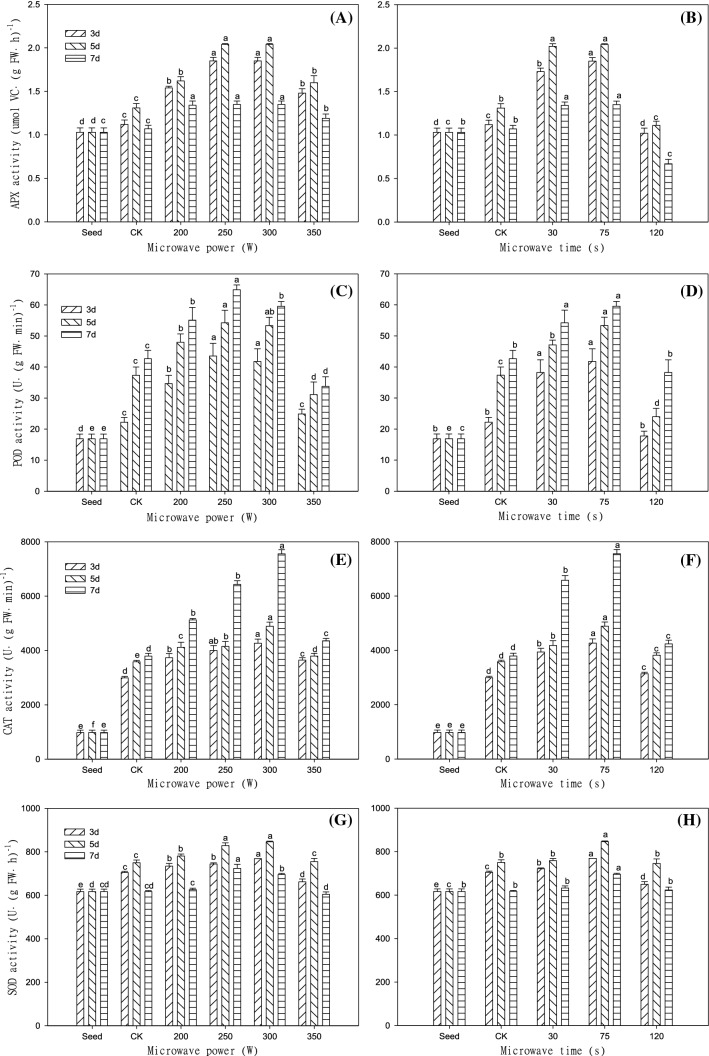
The activity of APX in seeds was 1.03 μmol VC·(g FW·h)−1. When treated with microwave at 300 W for 75 s, the APX activity in the 3- and 5-day-old sprouts was the strongest, and the APX activity of sprouts on the 5th day reached 2.04 μmol VC·(g FW·h)−1, which was 55.73% and 98.06% higher than that of the control and the seeds, respectively (Fig. 1A, B). After the treatment time of 120 s, the APX activity of sprouts for 3, 5 and 7 days was lower than that of the control. This suggests that long-term microwave radiation may cause enzyme mutations or inactivation. However, there was little difference between the seeds and the control group in APX activity, possibly because buckwheat itself had a well-organized self-defence mechanism to resist endogenous free radicals (Li et al. 2012).
Fig. 1.
Changes in the APX, POD, CAT and SOD activity of Tartary buckwheat sprouts in response to different microwave treatments. A, C, E, G: microwave time (75 s); B, D, F, H: microwave power (300 W).Values of the APX, POD, CAT and SOD activity with different microwave treatment levels shown with different lowercase letters are significantly different by ANOVA with Duncan's multiple range test at P < 0.05
The POD activity in seeds was 16.89 U·(g FW·min)−1. When treated with microwave at 250 W for 75 s, the POD activity of sprouts after 3, 5 and 7 days of germination was the highest, among which the POD activity of sprouts on the 7th day was 52.07% higher than that of the control, 3.84 times higher than that of the seeds, and the difference was significant (P < 0.05) (Fig. 1C, D). The POD activity significantly increased from 0 to 3 days, while Zhou et al. (2015) reported that the POD activity showed little change from 0 to 4 days, which may be the enhancement of enzyme activity in seeds by microwave treatment stimulated at the initial germination stage.
According to Fig. 1E, F, the CAT activity of Tartary buckwheat treated with microwave at 300 W for 75 s was the strongest on the 3rd, 5th and 7th days of germination. The CAT activity reached 7555.56 U·(g FW min)−1, 99.22% higher than that of the control and 7.73 times higher than that of seeds (300 W for 75 s), the difference between the different treatments was significant (P < 0.05). The CAT activity was enhanced by microwave stimulation, but the enzyme activity of the 120 s was significantly lower than that of the 75 s treatment (P < 0.05). Chen et al. (2009) also reported that appropriate microwave treatments could enhance the activity of CAT and other antioxidant enzymes. However, when seed germinated on the 3rd, 5th and 7th days, the CAT activity continued to increase. This is because in germination, the increase of H2O2 content in the sprout can further stimulate the improvement of CAT activity to remove excessive reactive oxygen species (Zheng et al. 2018a, b).
When microwave conditions were 300 W for 75 s, the SOD activity of sprouts after 5 days of germination reached 846.11 U·(g FW h)−1, higher than that both of the control and the seeds, and there was a significant difference between the different treatments (P < 0.05) (Fig. 1G, H). It reported high power and long-term microwave irradiation inhibited SOD activity and hindered seedling growth of wheat (Hao and Yang 2012). During 0 − 5 days of germination, APX and SOD activities were continuously enhanced and then decreased after reaching their maximum values on the 5th day (Fig. 1A, B, G, H), which was consistent with the research results reported by Zhou et al. (2015).
Total reducing power (TRP)
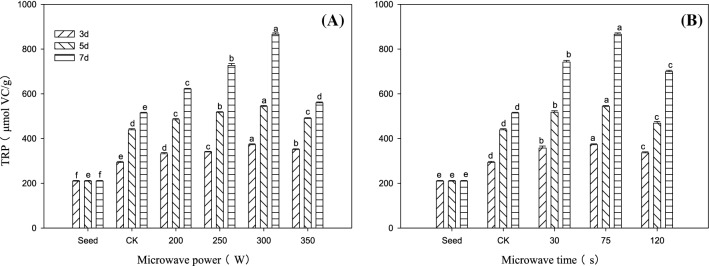
As shown in Fig. 2, under the microwave of 300 W for 75 s, the TRP of Tartary buckwheat sprouts germinated for 7 days reached 866.67 μmol VC·g−1, 4.12 times that of the seeds, and 68.53% higher than that of the control. The difference between different treatments was significant (P < 0.05).
Fig. 2.
Changes in the TRP of Tartary buckwheat sprouts in response to different microwave treatments. A: microwave time (75 s); B: microwave power (300 W). Values of the TRP with different microwave treatment levels shown with different lowercase letters are significantly different by ANOVA with Duncan' s multiple range test at P < 0.05
DPPH, ABTS, Hydroxyl and Superoxide anion RSA
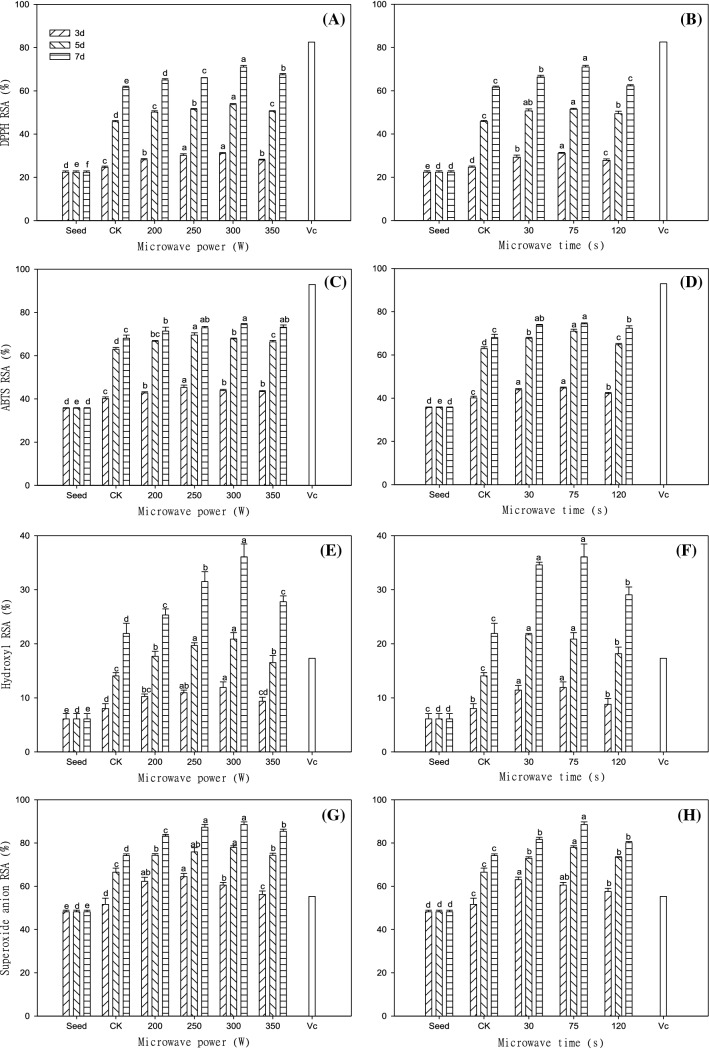
After microwave treatment at 300 W for 75 s, the DPPH RSA of sprouts at 3, 5 and 7 days was lower than that of VC. The DPPH RSA of sprouts after 7 days was 15.15% higher than that of the control group, and 3.18 times higher than that of seeds. There were significant differences between different treatments (P < 0.05). Using a microwave treatment of 350 W for 75 s and 300 W for 120 s, the DPPH RSA of the sprouts was 5.04% and 11.80%, which was lower than those treated at 300 W and 75 s microwave treatment, respectively. (Fig. 3A, B).
Fig. 3.
Changes in the DPPH, ABTS, Hydroxyl and Superoxide anion RSA of Tartary buckwheat sprouts in response to different microwave treatments. A, C, E, G: microwave time (75 s); B, D, F, H: microwave power (300 W). Values of the DPPH, ABTS, Hydroxyl and Superoxide anion RSA with different microwave treatment levels shown with different lowercase letters are significantly different by ANOVA with Duncan's multiple range test at P < 0.05
Under the microwave treatment of 300 W for 75 s, the ABTS RSA of sprouts of 3, 5 and 7 days was the highest but lower than that of VC. Among them, the ABTS RSA of sprouts that germinated for 7 days was 9.30% higher than that of the control group, and 2.08 times higher than that of seeds (Fig. 3C, D). There were significant differences between different treatments (P < 0.05). And it was higher than that reported by Zheng et al. (2018a, b). This may be because the biological effect of microwave significantly promoted total phenol and flavonoid content and enhanced the antioxidant mechanism of the sprouts.
As shown in Fig. 3E, F, under the condition of the power 300 W and time 75 s, the sprouts of days 3, 5 and 7 had the highest ability to remove •OH, and the ability to remove •OH was higher than that of VC on days 5 and 7. Among them, the •OH scavenging rate was 5.90 times that of seeds, 14.17% higher than that of the control group, and 24.16% and 29.84% higher than that of sprouts treated with a power of 300 W for 120 s and a power of 350 W for 75 s, respectively (7 d). Furthermore, there were significant differences between the different treatments (P < 0.05).
As can be seen in Fig. 3G, H, when treated with microwave at 300 W for 75 s, the Tartary buckwheat sprouts cultivated for days 3, 5 and 7 had the strongest ability to remove O2–. The scavenging rate of sprouts cultivated on 7th day reached 88.58%, which was 14.40% higher than that of the control and 1.84 times higher than that of seeds.
The TRP and DPPH, ABTS, O2– and •OH RSAs all increased because of the accumulation of phenols, flavonoids, vitamins and other antioxidants after Tartary buckwheat germination (Zheng et al. 2018a, b and Zhou et al. 2015). The antioxidant activity and free radical scavenging ability of phenolic substances are attributed to the reaction of oxygen free radicals and the phenolic hydroxyl groups on their molecular structures to form semi-quinone-type free radicals, which stops the chain reaction (Zheng et al. 2018a, b).
Conclusion
The appropriate microwave treatment power and time can significantly improve the activity of SOD, CAT, POD and APX in Tartary buckwheat sprouts and significantly enhance their DPPH, ABTS, O2– and •OH RSAs to improve the antioxidant ability of Tartary buckwheat sprouts. This study concludes that appropriate microwave radiation can improve antioxidant capacities in Tartary buckwheat sprouts.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 31772025) and the Anhui Natural Science Foundation (Grant No. 1808085MC93).
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aladjadjiyan A. Effect of microwave irradiation on seeds of lentils (lens culinaris, med.) Roman J Biophys. 2010;20(3):213–221. [Google Scholar]
- Aniszewska M, Słowiński K. Effects of microwave irradiation by means of a horn antenna in the process of seed extraction on Scots pine ( Pinus sylvestris L.) cone moisture content and seed germination energy and capacity. Eur J Forest Res. 2016;135(4):633–642. doi: 10.1007/s10342-016-0960-0. [DOI] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot-Lond. 2003;91(2):179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan JJ, Jagtap UB, Gaikwad NB, Dixit GB, Bapat VA. Total phenolics, flavonoids and antioxidant activity of Saptarangi (Salacia chinensis L.) fruit pulp. J Plant Biochem Biotechnol. 2013;22(4):409–413. doi: 10.1007/s13562-012-0169-3. [DOI] [Google Scholar]
- Chen W, Feng L, Shen Y, Su H, Li Y, Zhuang J, Zhang L. Zheng X (2013) Myricitrin inhibits acrylamide-mediated cytotoxicity in human Caco-2 cells by preventing oxidative stress. Biomed Res Int. 2013;2013:1–7. doi: 10.1155/2013/724183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, Jia JF, Han XL. Weak microwave can alleviate water deficit induced by osmotic stress in wheat seedlings. Planta. 2009;229(02):291–298. doi: 10.1007/s00425-008-0828-8. [DOI] [PubMed] [Google Scholar]
- Fikret Y, Manar T, Sebnem E, Sebnem K, Ozlem U. SOD, CAT, GR and APX enzyme activities in callus tissues of susceptible and tolerant eggplant varieties under salt stress. Res J Biotech. 2013;8(11):45–50. [Google Scholar]
- Hao YS, Yang LY. Effects of microwave pretreatment on seed germination and seedlings growth in wheat. J Shanxi Agric Sci (In China) 2012;40(06):608–612. [Google Scholar]
- Ji HB, Tang W, Zhou XL, Wu Y. Combined effects of blue and ultraviolet lights on the accumulation of flavonoids in Tartary buckwheat sprouts. Polish J Food Nutr Sci. 2016;66(2):93–98. doi: 10.1515/pjfns-2015-0042. [DOI] [Google Scholar]
- Kouchebagh SB, Rasouli P, Babaiy AH, Khanlou AR. Seed germination of pot marigold (Calendula officinalis L.) as affected by physical priming techniques. Int J Biosci. 2015;6(5):49–54. doi: 10.12692/ijb/6.5.49-54. [DOI] [Google Scholar]
- Li B, Li Y, Hu Q. Antioxidant activity of flavonoids from tartary buckwheat bran. Toxico Environ Chem. 2016;98(3–4):429–438. doi: 10.1080/02772248.2015.1123486. [DOI] [Google Scholar]
- Li XH, Park NI, Kim YB, Kim HH, Park CH, Wu Q, Park SU. Accumulation of flavonoids and expression of flavonoid biosynthetic genes in tartary and rice-tartary buckwheat. Process Biochem. 2012;47(12):2306–2310. doi: 10.1016/j.procbio.2012.09.009. [DOI] [Google Scholar]
- Ma Y, Wang P, Wang M, Sun M, Gu Z, Yang R. GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chem. 2019;270:593–601. doi: 10.1016/j.foodchem.2018.07.092. [DOI] [PubMed] [Google Scholar]
- Ma YY, Xiong YLL, Zhai JJ, Zhu HN, Dziubla T. Fractionation and evaluation of radical scavenging peptides from in vitro digests of buckwheat protein. Food Chem. 2010;118(3):582–588. doi: 10.1016/j.foodchem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam TG, Lee SM, Park JH, Kim DO, Ni B, Eom SH. Flavonoid analysis of buckwheat sprouts. Food Chem. 2015;170(170):97–101. doi: 10.1016/j.foodchem.2014.08.067. [DOI] [PubMed] [Google Scholar]
- Park BI, Kim J, Lee K, Lim T, Hwang KT. Flavonoids in common and tartary buckwheat hull extracts and antioxidant activity of the extracts against lipids in mayonnaise. J Food Sci Technol. 2019;56(5):2712–2720. doi: 10.1007/s13197-019-03761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu ZB, Guo JL, Zhang MM, Lei MY, Li ZL. Nitric oxide acts as a signal molecule in microwave pretreatment induced cadmium tolerance in wheat seedlings. Acta Physiol Plant. 2013;35(1):65–73. doi: 10.1007/s11738-012-1048-1. [DOI] [Google Scholar]
- Siddhuraju P, Becker K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Food Chem. 2007;101(1):10–19. doi: 10.1016/j.foodchem.2006.01.004. [DOI] [Google Scholar]
- Thwe AA, Kim Y, Li X, Kim YB, Park NI, Kim HH, Kim SJ, Park SU. Accumulation of phenylpropanoids and correlated gene expression in hairy roots of tartary buckwheat under light and dark conditions. Appl Biochem Biotechnol. 2014;174(7):2537–2547. doi: 10.1007/s12010-014-1203-9. [DOI] [PubMed] [Google Scholar]
- Wang SM, Wang JF, Guo YB. Microwave irradiation enhances the germination rate of Tartary buckwheat and content of some compounds in its sprouts. Polish J Food Nutr Sci. 2018;68(3):195–205. doi: 10.1515/pjfns-2017-0025. [DOI] [Google Scholar]
- Zheng CX, Hao JX, Song SH, Wang ZQ, Liu HJ. Effect of slightly acidic electrolyzed water on the bioactive compounds and antioxidant activity of tartary buckwheat sprouts. Food Sci (Beijing) 2018;39(4):20–25. [Google Scholar]
- Zheng CX, Nirasawa S, Song SH, Jiang ZQ, Liu HJ. The Effect of Slightly Acidic Electrolyzed Water on the Germination and Antioxidant Enzymes of Tartary Buckwheat Sprout. J Chin Inst Food Sci Technol. 2018;18(01):137–145. [Google Scholar]
- Zhou LY, Tey CY, Bingol G, Bi JF. Effect of microwave treatment on enzyme inactivation and quality change of defatted avocado puree during storage. Innov food sci emerg. 2016;37(A):61–67. doi: 10.1016/j.ifset.2016.08.002. [DOI] [Google Scholar]
- Zhou XL, Cheng SN, Yang YL, Zhou YM, Tang W, Zhang XJ, Wang Q, Li ZJ. Toward a novel understanding of buckwheat self-defensive strategies during seed germination and preliminary investigation on the potential pharmacological application of its malting products. J Med Plants Res. 2011;5(32):6946–6954. [Google Scholar]
- Zhou XL, Fang X, Zhou YM, Qian OY, Zhe L, Jun MA. Effect of magnetic field stimulation on flavonoid synthesis in tartary buckwheat(Fagopyrum tataricum Gaertn.) sprouts. Food Sci (Beijing) 2012;33(21):20–23. [Google Scholar]
- Zhou XL, Hao TF, Zhou YM, Tang W, Xiao Y, Meng XX, Fang X. Relationships between antioxidant compounds and antioxidant activities of tartary buckwheat during germination. J Food Sci Technol. 2015;52(4):2458–2463. doi: 10.1007/s13197-014-1290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]