Abstract
Immediate hypersensitivity reaction (IHR) can be divided into allergic- and non-allergic-mediated, while “anaphylaxis” is reserved for severe IHR. Clinically, true penicillin allergy is rare and most reported penicillin allergy is “spurious”. Penicillin-initiated anaphylaxis is possible to occur in skin test- and specific IgE-negative patients. The contact system is a plasma protease cascade initiated by activation of factor XII (FXII). Many agents with negative ion surface can activate FXII to drive contact system. Our data showed that penicillin significantly induced hypothermia in propranolol- or pertussis toxin-pretreated mice. It also caused a rapid and reversible drop in rat blood pressure, which did not overlap with IgE-mediated hypotension. These effects could be countered by a bradykinin-B2 receptor antagonist icatibant, and consistently, penicillin indeed increased rat plasma bradykinin. Moreover, penicillin not only directly activated contact system FXII-dependently, but also promoted bradykinin release in plasma incubated-human umbilical vein endothelial cells. In fact, besides penicillin, other beta-lactams also activated the contact system in vitro. Since the autoactivation of FXII can be affected by multiple-factors, plasma from different healthy individuals showed vastly different amidolytic activity in response to penicillin, suggesting the necessity of determining the potency of penicillin to induce individual plasma FXII activation. These results clarify that penicillin-initiated non-allergic anaphylaxis is attributed to contact system activation, which might bring more effective diagnosis options for predicting penicillin-induced fatal risk and avoiding costly and inappropriate treatment clinically.
Subject terms: Cell death and immune response, Coagulation system
Introduction
Anaphylaxis is a serious, life-threatening, generalized or systemic, allergy or non-allergic hypersensitivity with hypotension and vascular hyperpermeability as underlying symptoms1,2. Drugs are the most common anaphylaxis triggers in adults3–5. Betalactams, including penicillins and cephalosporins, are the most widely used antibiotics owing to their high safety profile, broad spectrum of activity, and low costs6. However, together with cephalosporins, penicillins are the antibiotics that most frequently provoke hypersensitivity reactions ranging from mild cutaneous symptoms to severe, life-threatening reactions7. Previous reports demonstrated that 42.6% of severe drug-induced anaphylaxis was caused by beta-lactam drugs8, and penicillin was the most frequent cause and accounted for approximately 75% of fatal anaphylactic cases in the United States9.
Penicillin, discovered by Alexander Fleming in 1928, is still one of the most widely prescribed antibiotics today. Clinically, approximately 10% of patients report a penicillin allergy (PenA), and its incidence of anaphylaxis has been estimated to be between 0.015 and 0.004%10,11. However, the reported PenA does not equate with true IgE-mediated allergy. In fact, true PenA is somewhat rare since only 1% of the general population is actually allergic to penicillin12. Most individuals thought to be PenA do not have detectable specific IgE (sIgE), and lack positive results from skin testing and oral challenge13–16. Only less than 10% of those with PenA histories are found to be at risk for acute PenA17. Some researchers ascribed the false attribution of PenA to IgE decline (disappearance) or viral rash misdiagnosis, while up to 90% of patients reporting PenA were found not to have a true allergy10,12. Obviously, it is unconvincing that such a high false rate is completely attributed to the above reasons. These data suggest that non-allergic mechanism(s) may be more common in the clinic.
The contact system is a plasma protease cascade initiated by activation of factor XII (FXII). Activated FXII (FXIIa) initiates two branches of downstream events that mediate the interface between coagulation and inflammation. The coagulation branch-activated FXI initiates fibrin formation and the inflammatory branch (also called kallikrein-kinin system)-activated prekallikrein cleaves the nonenzymatic cofactor high molecular weight kininogen (HK) to liberate oligopeptide bradykinin (BK)18. BK is involved in the regulation of inflammatory processes, vascular permeability, and blood pressure. Recent data have linked FXIIa-driven formation of BK and the downstream activation of the G-protein-coupled receptor B2 (B2R) signaling to anaphylaxis19,20. The name “contact system” comes from the mode of FXII being activated, as “contact” with negatively charged surfaces triggers FXII activation via conformational rearrangement. Activators that initiate FXIIa formation in vivo include polyphosphate, heparin, misfolded proteins, collagen, nucleic acids (DNA and RNA), oversulfated chondroitin sulfate and artificial surfaces21,22.
In the present study, we demonstrate for the first time that penicillin-driven FXII contact activation triggers kallikrein-kinin system, thus releasing BK to cause anaphylaxis. Our findings suggest that targeting contact system-produced BK or its downstream signaling is a promising strategy for prevention and treatment of anaphylaxis triggered by penicillin.
Materials and methods
Materials and reagents
Penicillin G sodium salt (1,550 U/mg) and other beta-lactams were from Solarbio Life Sciences (Beijing, China) and Yuanye Biotechnology Ltd. (Shanghai, China), respectively. Icatibant was from MedChemExpress (Monmouth Junction, NJ, USA). S-2302™ was from Chromogenix (Milano, Italy). BK ELISA kit was from DS Pharma Biomedical Co., Ltd. (Osaka, Japan). Kaolin, dextran sulfate sodium salt (DS; Mr ~ 500,000), propranolol, triprolidine, SB290157 and CV3988 were from Sigma-Aldrich (Darmstadt, German). PMX53 and pertussis toxin (PTX) were from GL Biochem Ltd. (Shanghai, China) and Enzo Life Sciences (Farmingdale, NY, USA), respectively. Citrate-anticoagulant standard human plasma and FXII-depleted plasma were from Boatman Biotech Co., Ltd. (Shanghai, China). Antibodies for human FXII and transferrin were from GeneTex Inc. (San Antonio, TX, USA). Shrimp tropomyosin (ST) and its IgE monoclonal antibody (mAb) were prepared as we previously described23. Human plasma was obtained from eight healthy volunteers.
Cells and animals
Primary human umbilical vein endothelial cells (HUVEC) were from Lonza (Basel, Switzerland). The male Balb/c mice (18–20 g) and SD rats (180–200 g) were purchased from Vital River Experimental Animal Services (Beijing, China) and housed in an SPF laboratory.
Anaphylactic shock assay
Anaphylactic shock was assessed by rectal thermometry24. To increase the severity of anaphylaxis, the mice were pretreated (i.v.) with propranolol (35 μg/mouse)25,26. Twenty minutes later, the mice were challenged (i.p.) with normal saline (NS) or penicillin (50 KU/mouse). Thirty minutes later, the rectal temperature was measured. For the BK antagonist experiment, icatibant (0.25 mM, 800 μL/mouse) was injected (i.p.) into the mice 10 min before the propranolol pretreatment.
The anaphylactic shock assay in PTX-pretreated mice was performed according to previously described procedures27. Briefly, the mice were injected (i.p.) with PTX (200 ng/mouse) on days 1 and 3. On day 7, the mice were injected (i.v.) with normal saline (NS) or penicillin (30 KU/mouse). Thirty minutes later, the rectal temperature was measured.
Evans blue extravasation assays
Evans blue extravasation assays in mouse hindpaws and rat dorsal skin were measured as previously described28,29.
Measurement of blood pressure and BK generation in rats
The rats were anesthetized and placed in a supine position. Catheters were placed into the carotid artery. Systolic arterial blood pressure was monitored in the left carotid artery with an arterial catheter connected to a polygraph (RM6240C, Chengdu, P.R. China). After equilibrating, the rats were injected (i.v.) with penicillin (1,250 KU/kg) or NS, and blood pressure was continuously monitored. For the antagonist experiment, rats were treated with icatibant (200 μg/kg, i.v.) 5 min prior to penicillin challenge.
For the determination of plasma BK, once penicillin (1,250 KU/kg) or NS was injected (i.v.) into the anesthetized rats, arterial blood was immediately collected and added to ice-cooled absolute ethanol. BK level was determined by ELISA.
The overlapping effect of penicillin- and antigen/IgE-caused hypotension was measured using a rat undergoing passive systemic anaphylaxis. To clarify the respective role of penicillin and antigen, we deliberately chose anti-ST IgE, a non-penicillin IgE mAb, to induce IgE/antigen hypotension. Rats were passively sensitized intravenously (i.v.) with 1 mg/kg of anti-ST IgE. Twenty-four hours later, the rats were challenged (i.v.) with ST (10 mg/kg) with or without penicillin (1,250 KU/kg), and the arterial blood pressure values were measured.
Amidolytic activity assay
Amidolytic activity was assayed according to previously described procedures30. Briefly, 100 μL of plasma was pretreated with 100 μL of penicillin at various concentrations (diluted by Tris buffer: 50 mM Tris–HCl, 0.117 M NaCl, pH 7.8) at 37 °C. Ten minutes later, 100 μL of the chromogenic substrate S-2302 (1.5 mg/mL) was added and further incubated at 37 °C for 30 min. The reaction mix was centrifuged at 3,000 × g for 5 min. Supernatant absorbance was monitored at 405 nm. Kaolin and DS were used as positive control activators of the contact system. Buffer alone was included as a negative control.
Determination of BK release in plasma incubated-human umbilical vein endothelial cells (PI-HUVEC)
The HUVEC were incubated with 10% standard human plasma in the presence of 20 μM Zn2+ at 37 °C for 1 h. The plasma was removed and the cells were washed twice. The obtained cells (PI-HUVEC) and non-PI-HUVEC were further incubated with penicillin at 37 °C for 30 min. Supernatant BK was determined by ELISA18,31.
Data presentation
The data reported as the mean ± SD from a representative experiment. All of the experiments reported in this work were repeated at least three times with the same pattern of results. The statistical analysis was performed using a one-way ANOVA. A student’s t-test was used when only two groups were compared. P < 0.05 was considered significant.
Ethic statement
Human plasma from healthy volunteers was obtained with written informed consent and all human studies were approved by the Ethic Review Board, Institute of Medicinal Plant Development of Chinese Academy of Medical Sciences. All the animal experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Care and Use Committee, Institute of Medicinal Plant Development of Chinese Academy of Medical Sciences. The informed consent form with official seal (the ethics approval number: 20180542x) for human blood collected from eight healthy volunteers. The animal ethics approval numbers are 20170728, 20171008, 20171234, 20180311 and 20180801. We confirm that all methods were performed in accordance with the relevant guidelines and regulations of our Ethic Review Board. Anesthetic drugs and all other necessary measures were used to reduce animal suffering during experimental procedures.
Results
Penicillin lowers rectal temperature in propranolol- or PTX-pretreated mice
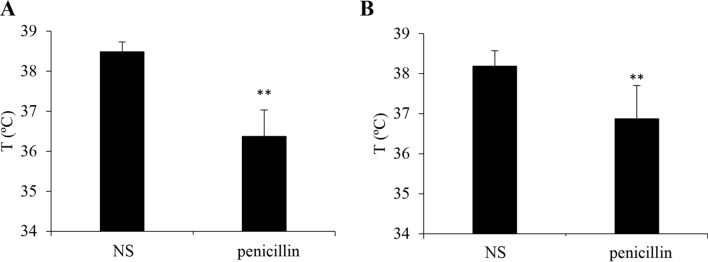
Given that penicillin can provoke severe and life-threatening anaphylaxis clinically, we first evaluated whether penicillin could cause anaphylactic shock (detected as hypothermia)7,24. Mice were made more sensitive to penicillin-induced shock by pretreating propranolol or PTX, which does not induce shock by itself but can augment the susceptibility of shock via enhancing vascular permeability25–27,32. Penicillin contributed to obvious hypothermia in both propranolol- and PTX-pretreated mice (Fig. 1), indicating that penicillin can also cause a non-allergic anaphylactic shock.
Figure 1.
Penicillin induces anaphylactic shock in propranolol- or PTX-pretreated mice (n = 8). (A) Penicillin-induced hypothermia in propranolol-pretreated mice. The mice were pretreated (i.v.) with propranolol (35 μg/mouse). Twenty minutes later, the mice were injected (i.p.) with normal saline (NS) or penicillin (50 KU/mouse). Thirty minutes later, the rectal temperature was measured. **P < 0.01 vs. NS. (B) Penicillin caused hypothermia in PTX-pretreated mice. The mice were injected (i.p.) with PTX (200 ng/mouse) on days 1 and 3. On day 7, the mice were injected (i.v.) with NS or penicillin (30 KU/mouse). Thirty minutes later, the rectal temperature was measured. **P < 0.01 vs. NS.
Penicillin increases microvascular permeability in a BK-dependent manner
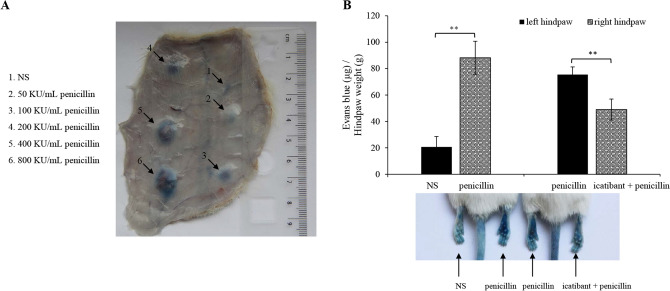
The local effect of penicillin on microvascular permeability was determined using the Evans blue extravasation assay. As shown in Fig. 2A, penicillin significantly (diameter of blue spot ≥ 5 mm) increased rat dorsal skin vasopermeability when the dose was ≥ 100 KU/mL (100 μL per spot). Moreover, it potently caused mouse plantar blue dye (right hindpaw) compared with NS (left hindpaw) (P < 0.01). To address the underlying mechanism, we next screened 6 antagonists (CV3988, SB290157, PMX53, triprolidine, fasudil and icatibant). The results showed that penicillin-increased microvascular permeability was independent of PAF, C3a, C5a, histamine and ROCK33 (data not shown), but could be countered by the B2R antagonist icatibant (Fig. 2B).
Figure 2.
Penicillin increases microvascular permeability in a BK-dependent manner. (A) Representative image of Evans blue extravasation of rat dorsal inboard skin induced by penicillin (n = 3). Rats were anesthetized and intracutaneously injected with 100 μL of penicillin at different dosages and then immediately intravenously injected with 5 mg/mL of Evans blue (1 mL). Ten minutes later, the rats were euthanized and the resultant blue spots in the dorsal inboard skin were scored. A diameter > 5 mm was considered positive. (B) Icatibant blocked penicillin-induced local vasopermeability (n = 8). Fifteen minutes after induction of anesthesia (50 mg/kg of pentobarbital), mice were intraplantarly injected with 10 μL of icatibant (0.5 mM) or normal saline (NS). Twenty minutes later, the mice were injected (i.v.) with 50 μL of 12.5 mg/mL Evans blue. Five minutes later, 10 μL of penicillin (400 KU/mL) or NS was administered by intraplantar injection in the paw. Thirty minutes later, the mice were euthanized. The paw tissues were collected and weighed. Evans blue was extracted by a 24 h incubation in formamide at 60 °C. The OD values were read at 620 nm. The concentration of the dye in the paw tissues was calculated from the standard curve of the Evans blue dye, and the dye content was expressed in microgram per gram of tissue. **P < 0.01.
Penicillin contributes to anaphylaxis by contact system activation (CSA) in rodents
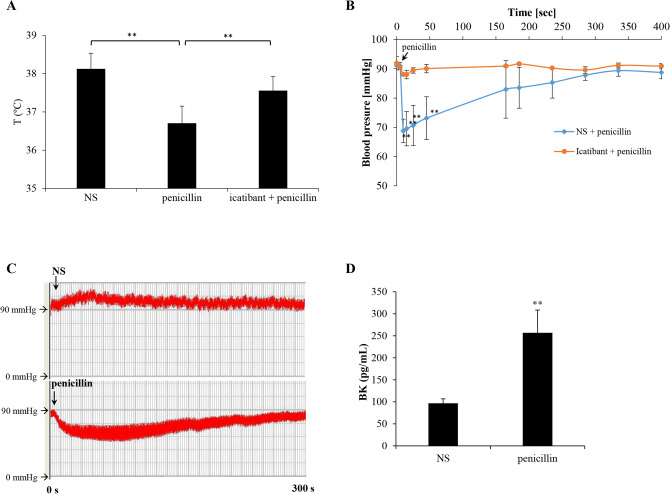
Based on the above results, we next evaluated whether icatibant could block penicillin-caused non-allergic anaphylaxis. First, we evaluated the effect of icatibant on penicillin-induced hypothermia in propranolol-pretreated mice. As expected, icatibant indeed significantly countered penicillin-caused mouse hypothermia (P < 0.01; Fig. 3A). Given that acute hypotension is an underlying symptom of anaphylaxis34, the effect of penicillin on rat arterial blood pressure was then determined. Unlike intra-arterial administration of heparin19, intravenous injection of penicillin could trigger a rapid and transient drop in systemic arterial blood pressure. Pretreatment with icatibant almost completely blocked penicillin-caused hypotension (Fig. 3B,C). Along with the hypotension, plasma BK was markedly increased after penicillin challenge (Fig. 3D). These findings demonstrate that BK plays a crucial role in penicillin-initiated non-allergic anaphylaxis. In addition, to determine a possible overlapping effect of penicillin- and antigen/IgE-caused hypotension, SD rats were sensitized passively with a non-penicillin IgE (anti-ST IgE). The overlapping effect assay was carried out by intravenously injecting penicillin and antigen (ST) into the ST-IgE-sensitized rats. As a result (Table 1), penicillin and ST could respectively cause hypotension in normal and antigen/IgE-sensitized rats. Unexpectedly, penicillin did not cooperate with ST to trigger more drastic hypotension in antigen/IgE-sensitized rats.
Figure 3.
Penicillin contributes to anaphylaxis by activating the contact system in rodents. (A) Penicillin-induced hypothermia could be countered by icatibant in propranolol-pretreated mice (n = 8). **P < 0.01. (B) Penicillin induced a rapid and reversible drop in blood pressure in a BK-dependent manner (n = 6). The rats were pretreated with icatibant (200 μg/kg, i.v.) or normal saline (NS). Five minutes later, penicillin (1,250 KU/kg) was injected (i.v.) and the arterial blood pressure was measured in NS-pretreated (blue line) or icatibant-pretreated (orange line) rats. **P < 0.01 vs. icatibant + penicillin. (C) Typical tracing showing that penicillin induced a rapid and reversible drop in rat blood pressure. The rats were injected (i.v.) with NS or penicillin (1,250 KU/kg) and the arterial blood pressure was constantly monitored. (D) Penicillin significantly increased BK release in rat plasma. BK concentration was determined by a commercial ELISA kit. **P < 0.01 vs. NS.
Table 1.
Mean arterial blood pressure values of normal or anti-shrimp tropomyosin (ST) IgE monoclonal antibody-sensitized rats in response to ST and/or penicillin (n = 5).
| Challenge | Time (s) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 10 | 30 | 60 | 120 | 180 | 240 | 300 | |
| Penicillin (1,250 KU/kg)a | 91.2 ± 0.1 | 68.8 ± 4.0 | 70.1 ± 6.9 | 74.8 ± 7.3 | 82.9 ± 9.9 | 83.5 ± 7.0 | 85.3 ± 5.3 | 87.8 ± 1.8 |
| ST (10 mg/kg)b | 91.3 ± 0.8 | 71.3 ± 4.0 | 75.0 ± 3.6 | 78.8 ± 3.3 | 84.9 ± 4.4 | 85.6 ± 3.0 | 86.4 ± 3.5 | 88.7 ± 2.2 |
| ST (10 mg/kg) + penicillin (1,250 KU/kg)b | 90.6 ± 0.6 | 71.2 ± 4.4 | 71.7 ± 3.1 | 75.6 ± 2.1 | 81.2 ± 1.9 | 82.8 ± 3.1 | 86.5 ± 2.5 | 87.6 ± 2.3 |
aChallenge in normal rats.
bChallenge in anti-ST IgE monoclonal antibody-sensitized rats.
Penicillin activates the contact system in an FXII-dependent manner
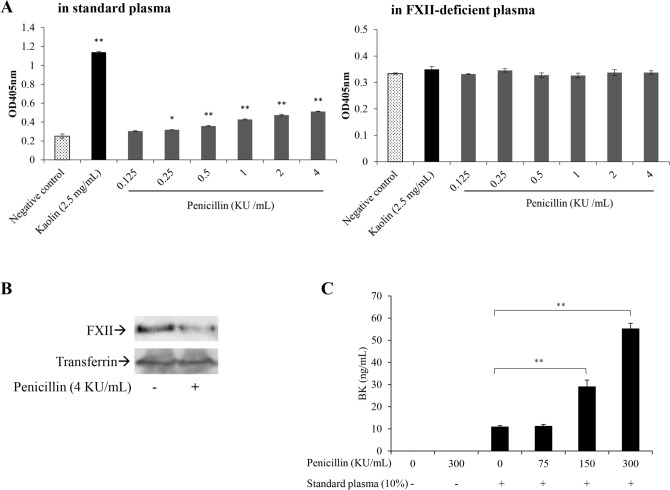
Direct activation of the contact system by penicillin was further investigated in vitro. Amidolytic activity was assessed in the standard plasma and FXII-deficient plasma by the addition of the S-2302 chromogenic substrate that can be hydrolyzed by plasma kallikrein. Similarly to the FXII activator kaolin, penicillin directly activated the contact system in standard human plasma, and this effect almost disappeared in FXII-deficient plasma (Fig. 4A). Consistently, owing to being converted into FXIIa consisting of a 52-kD heavy chain and a 28-kD serine protease domain, prototypical FXII (80-kD) in penicillin treated-plasma was also significantly decreased (Fig. 4B). BK, a short-lived bioactive peptide35, is the terminal product of CSA cascade. Given that the entire BK-forming cascade can be assembled and activated along the surface of endothelial cells36,37, HUVEC were incubated with standard human plasma and then the plasma was removed. The obtained PI-HUVEC, which had combined all elements for BK generation, could be used for the measurement of BK in vitro. As shown in Fig. 4C, unlike non-PI-HUVEC, PI-HUVEC could also release a small quantity of BK without penicillin. In contrast to non-PI-HUVEC, penicillin markedly promoted BK release in the PI-HUVEC in a concentration-dependent manner.
Figure 4.
Penicillin activates the contact system in an FXII-dependent manner. (A) Penicillin-induced CSA in standard or FXII-deficient human plasma. 100 μL of plasma was pretreated with 100 μL of penicillin at various concentrations (diluted by Tris buffer: 50 mM Tris–HCl, 0.117 M NaCl, pH 7.8) at 37 °C. Ten minutes later, 100 μL of the chromogenic substrate S-2302 (1.5 mg/mL) was added and further incubated at 37 °C for 30 min. The reaction mix was centrifuged at 3,000 × g for 5 min. Supernatant absorbance was monitored at 405 nm. Kaolin was used as a positive control of the contact system. Buffer alone was included as the negative control. *P < 0.05 and **P < 0.01 vs. negative control. (B) Plasma prototypical FXII level decreased after penicillin treatment. Standard human plasma was incubated with or without 4 KU/mL penicillin at 37 °C for 30 min and analyzed for FXII determination by western blotting. Transferrin was used as the internal reference. Full-length blots and the detailed information of the used antibodies were presented in Supplementary file 1. (C) Penicillin induced BK release in PI-HUVEC. HUVEC were incubated with 10% standard human plasma in the presence of 20 μM Zn2+ at 37 °C for 1 h. The plasma was removed and the cells were washed twice. PI-HUVEC and non-PI-HUVEC were further incubated with penicillin at 37 °C for 30 min. Supernatant BK was determined by ELISA. **P < 0.01.
Plasma from different individuals has distinct amidolytic activity
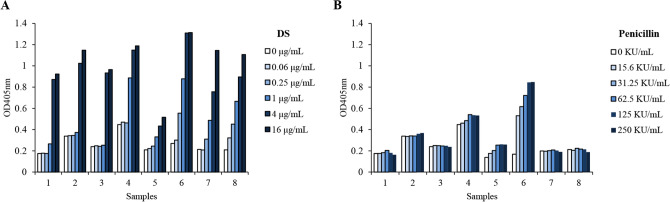
The autoactivation of FXII-mediated CSA can be affected by various factors, including FXII38 and its endogenous inhibitor C1INH levels39, ionic environment (especially Zn2+ concentration18,40 or the negative charge density of FXII activators41), etc. Thus, we reasoned that plasma from different individuals had distinct amidolytic activity. To confirm this point, we assayed respective amidolytic activity in plasma from volunteers. As shown in Fig. 5, eight plasma samples (nos. 1–8) showed a consistent concentration–response to DS, including a relatively weak response of no. 5 (Fig. 5A). In contrast to DS, the majority responsiveness to penicillin was lower, except for no. 6. And nos. 1, 2, 3, 7 and 8 did not change significantly after penicillin treatment (≤ 250 KU/mL). Particularly, nos. 4 and 6, who had positive skin-test histories, reacted differently in response to penicillin: no. 4 had a high basal level but low sensitivity, while no. 6 had a low basal level but high sensitivity (Fig. 5B). These findings obviously indicate that the activation level of the contact system varies with diverse activators (e.g., penicillin and DS).
Figure 5.
Plasma from different individuals has distinct amidolytic activity in response to DS (A) and penicillin (B). Human plasma was obtained from 8 healthy volunteers aged 20–54 years. 100 μL of plasma was pretreated with 100 μL of penicillin at various concentrations (diluted by Tris buffer: 50 mM Tris–HCl, 0.117 M NaCl, pH 7.8) at 37 °C. Ten minutes later, 100 μL of the chromogenic substrate S-2302 (1.5 mg/mL) was added and further incubated at 37 °C for 30 min. The reaction mix was centrifuged at 3,000 × g for 5 min. Supernatant absorbance was monitored at 405 nm.
Effects of other beta-lactams on CSA
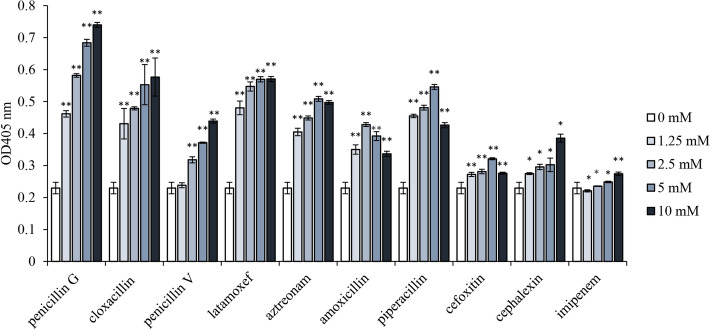
To date, beta-lactams are the most successful chemical class of antibiotic widely used in the clinic to treat infections in humans42. They are mainly divided into 6 subclasses including penicillins (e.g., penicillin G, penicillin V, cloxacillin, amoxicillin and piperacillin), cephalosporins (e.g., cephalexin), oxacephems (e.g., latamoxef), single-ring beta-lactams (e.g., aztreonam), cephamycins (e.g., cefoxitin), and carbapenems (e.g., imipenem). We found that the effects of different beta-lactams on plasma amidolytic activity were various. Penicillin G was found to be the most effective, and cloxacillin, latamoxef and piperacillin had similar impacts. Other beta-lactams were found to be less effective (Fig. 6).
Figure 6.
Effects of 10 beta-lactams on the amidolytic activity in standard human plasma. Beta-lactam antibiotics were incubated with standard human plasma at 37 °C for 10 min. Amidolytic activity in plasma was assayed using the chromogenic substrate S-2302. **P < 0.01 vs. negative control (0 mM).
Discussion
Immediate hypersensitivity reaction (IHR) can be divided into allergic- and non-allergic-mediated43. According to the World Allergy Organization proposition, “anaphylaxis” is reserved for severe IHR whose symptoms include hypotension, vascular leakage, or even cardiac arrhythmia and bronchial constriction in severe cases20,44. Previous studies have demonstrated that the FXII-driven contact system critically contributes to the pathogenesis of anaphylaxis in murine models20. Clinically, anaphylaxis is associated with transient decreased plasma FXII, suggesting that the factor is consumed. The severity of anaphylaxis is correlated with the intensity of CSA and BK formation20. The abnormal production of BK leads to anaphylaxis and angioedema via its ability to increase inflammation and vessel permeability21,45. There are common triggers for anaphylactic reactions such as food, medications or insect venom, of which 44–57% of the fatal anaphylaxis was triggered by medications, far more than other inducements3,46–48. The most common lethal reasons for fatal anaphylaxis are asphyxia and shock, which can be exactly caused by BK49–52.
An established concept is that penicillin can serve as a complete antigen to induce sIgE through irreversibly forming drug-protein conjugates in vivo53,54. Upon a secondary challenge, the penicillin-protein conjugate crosslinks its sIgE which bound to the high affinity receptor, FcɛRI, and leads to the degranulation of mast cells and basophils (Fig. 7), thus inducing type I hypersensitivity (e.g., anaphylaxis). In fact, true PenA is rare clinically, and most reported PenA is “spurious”55,56. Moreover, penicillin-induced IHR is possible to occur in both skin test- and sIgE-negative patients57,58, suggesting that a non-allergic mechanism exists. In fact, although penicillin could not directly induce human mast cell LAD2 degranulation in vitro (data not shown), it caused non-allergic anaphylaxis (Figs. 1, 3B). Our data showed that penicillin increased microvascular permeability, which could be countered by a B2R antagonist icatibant, rather than other antagonists (e.g., triprolidine and fasudil33) (Fig. 2B). Moreover, icatibant could also block penicillin induced-anaphylactic shock (Fig. 3A) and hypotension (Fig. 3B,C). It is worth mentioning that heparin, an endogenous FXII activator, causes hypotension only through intra-arterial application19, which may be attributed to its rapid catabolism in blood circulation. In the present study, we found that penicillin triggered hypotension via both intra-arterial and intravenous injection. Given the fact that intra-arterial application of penicillin, an exogenous stimulus, is clinically meaningless, only the results of intravenous challenge were shown. In line with the in vivo result (Fig. 3D), in vitro study indicated that penicillin initiated CSA through the activation of FXII (Fig. 4A,B) and finally led to BK formation (Fig. 4C). Together, penicillin-initiated non-allergic anaphylaxis was attributed to CSA in which FXIIa activates prekallikrein leading to BK release via cleavage of its precursor HK.
Figure 7.
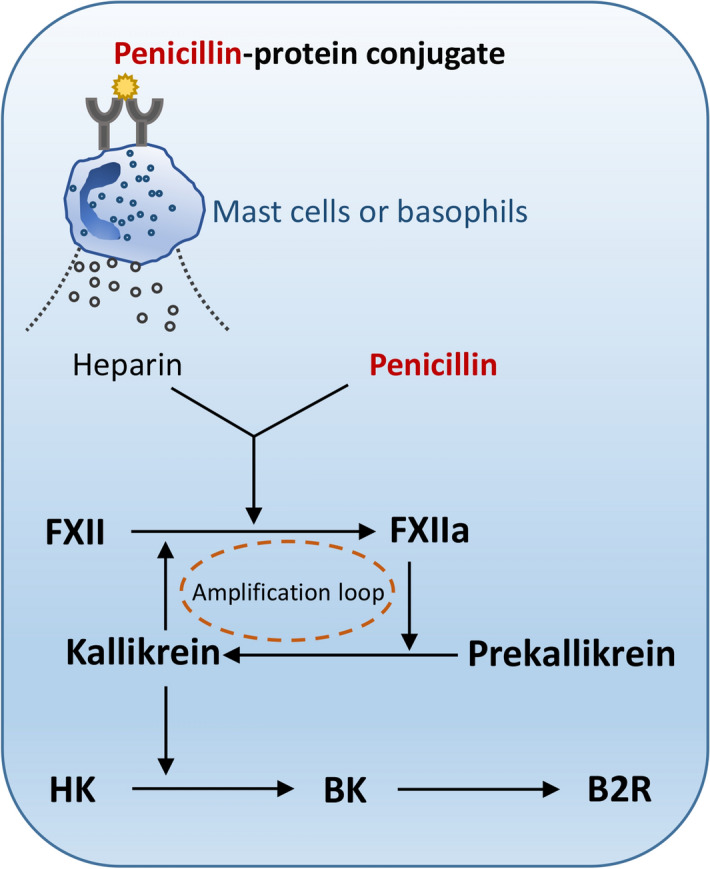
Penicillin triggers allergic- and non-allergic-mediated IHR via the FXIIa-BK-B2R axis.
The fact that penicillin can trigger both IgE- and non-allergic-mediated anaphylaxis raises the reasonable speculation about whether penicillin-induced severe or fatal anaphylaxis results from an overlapping effect. Unexpectedly, penicillin did not cooperate with antigen to trigger more drastic hypotension in antigen/IgE-sensitized rats (Table 1). In allergen/IgE-mediated type I hypersensitivity, mast cell or basophil-derived heparin can also initiate FXII contact activation and subsequently generate BK, which binds to B2R, triggering anaphylaxis19,20. Obviously, both allergen/IgE- and non-allergic-mediated anaphylaxis have the same pathway, namely the FXIIa-BK-B2R axis. Perhaps it is because the same axis that the overlapping effect did not occur. In addition, only one dose of penicillin was used in the rat blood pressure test due to the fact that penicillin caused hypotension in a non-dose-dependent manner (data not shown), which is similar to heparin19. As we know, FXIIa cleaves prekallikrein to form active kallikrein, which in turn reciprocally activates more FXII, forming a powerful activation amplification loop (Fig. 7). It is because of the “positive feedback loop” that, once initiated, the penicillin-induced cascade reaction reaches completion independent of the dosage of penicillin. In this context, other factors (e.g., plasma FXII or C1INH level, etc.) may be more crucial for reaction intensity.
FXII autoactivation can be influenced by multi-factors, such as FXII38 and C1INH levels39, ionic milieu18,40, or the negative charge density of FXII activators41, etc. Indeed, our data also showed that plasma from different human individuals had vastly different amidolytic activity in response to penicillin (Fig. 5B and Supplementary file 2), of which high responders might be the potential anaphylaxis sufferer (e.g., no. 6 in Fig. 5B and no. 5 in Supplementary file 2). In fact, some clinical evidences also support our findings. For example, penicillin-induced IHR was much more likely with parenteral administration than oral administration58. Angioedema, BK-mediated swelling45, is also common in penicillin-induced IHR59,60. Perhaps, not coincidentally, Caucasians, whose FXII levels are significantly higher than Japanese61, suffer from more penicillin-induced IHR compared with Asians including Japanese60.
Given that true PenA is rare clinically and the FXIIa-BK-B2R axis is the same pathway for penicillin-triggered allergic- and non-allergic-IHR (Fig. 7)19, compared with the commonly used skin test or sIgE assay, it is perhaps more effective to determine the potency of penicillin (or beta-lactams) to induce individual plasma FXII activation or BK formation for predicting the risk of penicillin-induced IHR. We notice that the World Health Organization has established a “first international standard for FXII, Plasma, human”62, which also ought to be beneficial for predicting the risk of penicillin-induced IHR, even including that of other beta-lactams (e.g., penicillin G, cloxacillin, latamoxef, piperacillin and aztreonam, etc.; Fig. 6). Additionally, in the same volunteer, the degree of FXII activation in response to penicillin at the same concentrations is disparate over time (no. 5 in Fig. 5 vs. Supplementary file 2), which might be attributed to the change of plasma milieus. Thus, instant prediction, rather than long-term predication, may be available for predicting the IHR risk of penicillin.
In summary, our study demonstrates, for the first time, that as an activator of the FXII-driven contact system, penicillin can lead to hypersensitivity reactions in rodent models, which can be hampered by icatibant, a B2R pharmacological inhibitor. These findings not only warrant further exploration of the penicillin-driven contact system as a source of therapeutic targets for treatment of anaphylaxis triggered by penicillin, but also might bring more effective diagnosis options for the prediction of penicillin-induced fatal risk.
Supplementary information
Acknowledgements
This study was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2016-I2M-3-015), National Natural Science Foundation of China (Nos. 81601385 and 81873066), and the Open Research Fund of State Key Laboratory Breeding Base of Systematic research, development and Utilization of Chinese Medicine Resources.
Author contributions
Y.G., C.P. and Y.Q. contributed to the design of the study. Y.G. and Y.Q. participated in manuscript writing and editing. Y.H., X.Z. and Q.F. carried out the main experiments. R.Q. and R.H. were involved in data collection and generation. R.C. contributed to data analysis.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cheng Peng, Email: pengchengchengdu@126.com.
Yun Qi, Email: yqi@implad.ac.cn.
Supplementary information
is available for this paper at 10.1038/s41598-020-71083-x.
References
- 1.Simons FE, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ. J. 2014;7:1–19. doi: 10.1186/1939-4551-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura T, Murata T. Regulation of vascular permeability in anaphylaxis. Br. J. Pharmacol. 2018;175:2538–2542. doi: 10.1111/bph.14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J. Allergy Clin. Immunol. 2009;123:434–442. doi: 10.1016/j.jaci.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 4.González-Pérez A, Aponte Z, Vidaurre CF, Rodríguez LA. Anaphylaxis epidemiology in patients with and patients without asthma: A United Kingdom database review. J. Allergy Clin. Immunol. 2010;125:1098–1104. doi: 10.1016/j.jaci.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Wood RA, et al. Anaphylaxis in America: The prevalence and characteristics of anaphylaxis in the United States. J. Allergy Clin. Immunol. 2014;133:461–467. doi: 10.1016/j.jaci.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Moreno E, et al. Using β-lactam antibiotics in patients with a history of β-lactam allergy: Current concepts. Pol. Arch. Intern. Med. 2017;127:540–549. doi: 10.20452/pamw.4066. [DOI] [PubMed] [Google Scholar]
- 7.Romano A, Caubet JC. Antibiotic allergies in children and adults: From clinical symptoms to skin testing diagnosis. J. Allergy Clin. Immunol. Pract. 2014;2:3–12. doi: 10.1016/j.jaip.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Renaudin JM, Beaudouin E, Ponvert C, Demoly P, Moneret-Vautrin DA. Severe drug-induced anaphylaxis: Analysis of 333 cases recorded by the Allergy Vigilance Network from 2002 to 2010. Allergy. 2013;68:929–937. doi: 10.1111/all.12168. [DOI] [PubMed] [Google Scholar]
- 9.Neugut AI, Ghatak AT, Miller RL. Anaphylaxis in the United States: An investigation into its epidemiology. Arch. Intern. Med. 2001;161:15–21. doi: 10.1001/archinte.161.1.15. [DOI] [PubMed] [Google Scholar]
- 10.Patterson RA, Stankewicz HA. Penicillin, Allergy. Treasure Island: StatPearls Publishing, LLC; 2018. [PubMed] [Google Scholar]
- 11.Idsoe, O., Guthe, T., Willcox, R.R. & Weck, A.L. de. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull. World Health Organ.38, 159–188 (1968). [PMC free article] [PubMed]
- 12.Trubiano JA, Adkinson NF, Phillips EJ. Penicillin allergy is not necessarily forever. JAMA. 2017;318:82–83. doi: 10.1001/jama.2017.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hjortlund J, Mortz CG, Skov PS, Bindslev-Jensen C. Diagnosis of penicillin allergy revisited: The value of case history, skin testing, specific IgE and prolonged challenge. Allergy. 2013;68:1057–1064. doi: 10.1111/all.12195. [DOI] [PubMed] [Google Scholar]
- 14.Macy E. Penicillin allergy: Optimizing diagnostic protocols, public health implications, and future research needs. Curr. Opin. Allergy Clin. Immunol. 2015;15:308–313. doi: 10.1097/ACI.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 15.Arroliga ME, et al. A pilot study of penicillin skin testing in patients with a history of penicillin allergy admitted to a medical ICU. Chest. 2000;118:1106–1108. doi: 10.1378/chest.118.4.1106. [DOI] [PubMed] [Google Scholar]
- 16.Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J. Allergy Clin. Immunol. Pract. 2013;1:258–263. doi: 10.1016/j.jaip.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Gadde J, Spence M, Wheeler B, Adkinson NF., Jr Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:2456–2463. [PubMed] [Google Scholar]
- 18.Schmaier AH. The contact activation and kallikrein/kinin systems: Pathophysiologic and physiologic activities. J. Thromb. Haemost. 2016;14:28–39. doi: 10.1111/jth.13194. [DOI] [PubMed] [Google Scholar]
- 19.Oschatz C, et al. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity. 2011;34:258–268. doi: 10.1016/j.immuni.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Sala-Cunill A, et al. Plasma contact system activation drives anaphylaxis in severe mast cell-mediated allergic reactions. J. Allergy Clin. Immunol. 2015;135:1031–1043. doi: 10.1016/j.jaci.2014.07.057. [DOI] [PubMed] [Google Scholar]
- 21.Bender L, Weidmann H, Rose-John S, Renné T, Long AT. Factor XII-driven inflammatory reactions with implications for anaphylaxis. Front. Immunol. 2017;8:1115. doi: 10.3389/fimmu.2017.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naudin C, Burillo E, Blankenberg S, Butler L, Renné T. Factor XII contact activation. Semin. Thromb. Hemost. 2017;43:814–826. doi: 10.1055/s-0036-1598003. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, et al. The Three-Herb Formula Shuang-Huang-Lian stabilizes mast cells through activation of mitochondrial calcium uniporter. Sci. Rep. 2017;7:38736. doi: 10.1038/srep38736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strait RT, Morris SC, Yang M, Qu X, Finkelman FD. Pathways of anaphylaxis in the mouse. J. Allergy Clin. Immunol. 2002;109:658–668. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 25.Khodoun M, et al. Peanuts can contribute to anaphylactic shock by activating complement. J. Allergy Clin. Immunol. 2009;123:342–351. doi: 10.1016/j.jaci.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang W, et al. β2-Adrenoceptor blockade deteriorates systemic anaphylaxis by enhancing hyperpermeability in anesthetized mice. Allergy Asthma Immunol. Res. 2018;10:52–61. doi: 10.4168/aair.2018.10.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, et al. Basophil activation through ASGM1 stimulation triggers PAF release and anaphylaxis-like shock in mice. Eur. J. Immunol. 2014;44:2468–2477. doi: 10.1002/eji.201344144. [DOI] [PubMed] [Google Scholar]
- 28.McNeil BD, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519:237–241. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y, et al. Shuang-Huang-Lian injection induces an immediate hypersensitivity reaction via C5a but not IgE. Sci. Rep. 2018;8:3572. doi: 10.1038/s41598-018-21843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kishimoto TK, et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N. Engl. J. Med. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan AP, Joseph K, Silverberg M. Pathways for bradykinin formation and inflammatory disease. J. Allergy Clin. Innumol. 2002;109:195–209. doi: 10.1067/mai.2002.121316. [DOI] [PubMed] [Google Scholar]
- 32.Ji N, Rao N, Guentzel NM, Arulanandam BP, Forsthuber TG. Anaphylaxis and mortality induced by treatment of mice with anti-VLA-4 antibody and pertussis toxin. J. Immunol. 2011;186:2750–2756. doi: 10.4049/jimmunol.1000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han J, et al. Involvement of histamine and RhoA/ROCK in penicillin immediate hypersensitivity reactions. Sci. Rep. 2016;6:33192. doi: 10.1038/srep33192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bircher AJ, Auerbach M. Hypersensitivity from intravenous iron products. Immunol. Allergy Clin. N. Am. 2014;34:707–723. doi: 10.1016/j.iac.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Murphey LJ, Hachey DL, Oates JA, Morrow JD, Brown NJ. Metabolism of bradykinin In vivo in humans: Identification of BK1-5 as a stable plasma peptide metabolite. J. Pharmacol. Exp. Ther. 2000;294:263–269. [PubMed] [Google Scholar]
- 36.Pathak M, Kaira BG, Slater A, Emsley J. Cell receptor and cofactor interactions of the contact activation system and factor XI. Front. Med. (Lausanne) 2018;5:66. doi: 10.3389/fmed.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan AP, Ghebrehiwet B. The plasma bradykinin-forming pathways and its interrelationships with complement. Mol. Immunol. 2010;47:2161–2169. doi: 10.1016/j.molimm.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Han Y, et al. Normal range and genetic analysis of coagulation factor XII in the general Chinese population. Thromb. Res. 2015;136:440–444. doi: 10.1016/j.thromres.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Han ED, MacFarlane RC, Mulligan AN, Scafidi J, Davis AE. Increased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptor. J. Clin. Investig. 2002;109:1057–1063. doi: 10.1172/JCI14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mutch NJ, Waters EK, Morrissey JH. Immobilized transition metal ions stimulate contact activation and drive factor XII-mediated coagulation. J. Thromb. Haemost. 2012;10:2108–2115. doi: 10.1111/j.1538-7836.2012.04890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller F, Renné T. Novel roles for factor XII-driven plasma contact activation system. Curr. Opin. Hematol. 2008;15:516–521. doi: 10.1097/MOH.0b013e328309ec85. [DOI] [PubMed] [Google Scholar]
- 42.Foster TJ. Can β-lactam antibiotics be resurrected to combat MRSA? Trends Microbiol. 2018;27:26–38. doi: 10.1016/j.tim.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Johansson SG, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813–824. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- 44.Johansson SG, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 45.Obtułowicz K. Bradykinin-mediated angioedema. Pol. Arch. Med. Wewn. 2016;126:76–85. doi: 10.20452/pamw.3273. [DOI] [PubMed] [Google Scholar]
- 46.Pumphrey RS. Fatal anaphylaxis in the UK, 1992–2001. Novartis Found Symp. 2004;257:116–128. [PubMed] [Google Scholar]
- 47.Pumphrey R. Anaphylaxis: Can we tell who is at risk of a fatal reaction? Curr. Opin. Allergy Clin. Immunol. 2004;4:285–290. doi: 10.1097/01.all.0000136762.89313.0b. [DOI] [PubMed] [Google Scholar]
- 48.Poulos LM, Waters AM, Correll PK, Loblay RH, Marks GB. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993–1994 to 2004–2005. J. Allergy Clin. Immunol. 2007;120:878–884. doi: 10.1016/j.jaci.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 49.Lee JK, Vadas P. Anaphylaxis: Mechanisms and management. Clin. Exp. Allergy. 2011;41:923–938. doi: 10.1111/j.1365-2222.2011.03779.x. [DOI] [PubMed] [Google Scholar]
- 50.Kaplan AP. The bradykinin-forming cascade: A historical perspective. Chem. Immunol. Allergy. 2014;100:205–213. doi: 10.1159/000358739. [DOI] [PubMed] [Google Scholar]
- 51.Banerji A. Hereditary angioedema: Classification, pathogenesis, and diagnosis. Allergy Asthma Proc. 2011;32:403–407. doi: 10.2500/aap.2011.32.3492. [DOI] [PubMed] [Google Scholar]
- 52.Christie G, Kitteringham NR, Park BK. Drug–protein conjugates-XIII. The disposition of the benzylpenicilloyl hapten conjugated to albumin. Biochem. Pharmacol. 1987;36:3379–3385. doi: 10.1016/0006-2952(87)90314-5. [DOI] [PubMed] [Google Scholar]
- 53.Castells, M., Khan, D. A. & Phillips, E. J. Penicillin Allergy. N. Engl. J. Med.381, 2338–2351 (2019). [DOI] [PubMed]
- 54.Chang C, Mahmood MM, Teuber SS, Gershwin ME. Overview of penicillin allergy. Clin. Rev. Allergy Immunol. 2012;43:84–97. doi: 10.1007/s12016-011-8279-6. [DOI] [PubMed] [Google Scholar]
- 55.Krishna MT, et al. Enhancing antibiotic stewardship by tackling "spurious" penicillin allergy. Clin. Exp. Allergy. 2017;47:1362–1373. doi: 10.1111/cea.13044. [DOI] [PubMed] [Google Scholar]
- 56.Torres MJ, et al. Controlled administration of penicillin to patients with a positive history but negative skin and specific serum IgE tests. Clin. Exp. Allergy. 2002;32:270–276. doi: 10.1046/j.1365-2222.2002.01296.x. [DOI] [PubMed] [Google Scholar]
- 57.Trubiano JA, Adkinson NF, Phillips EJ. Persistence of penicillin allergy-reply. JAMA. 2017;318:1714–1715. doi: 10.1001/jama.2017.13763. [DOI] [PubMed] [Google Scholar]
- 58.Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498–2505. doi: 10.1001/jama.285.19.2498. [DOI] [PubMed] [Google Scholar]
- 59.Meng J, Thursfield D, Lukawska JJ. Allergy test outcomes in patients self-reported as having penicillin allergy: Two-year experience. Ann. Allergy Asthma Immunol. 2016;117:273–279. doi: 10.1016/j.anai.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 60.Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc. 2014;35:489–494. doi: 10.2500/aap.2014.35.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon EM, Donaldson VH, Saito H, Su E, Ratnoff OD. Reduced titers of Hageman factor (factor XII) in orientals. Ann. Intern. Med. 1981;95:697–700. doi: 10.7326/0003-4819-95-6-697. [DOI] [PubMed] [Google Scholar]
- 62.Wilmot HV, Hockley J, Rigsby P, Gray E. Establishment of the World Health Organization first international standard for Factor XII, plasma, human. Front. Med. (Lausanne) 2018;5:46. doi: 10.3389/fmed.2018.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.