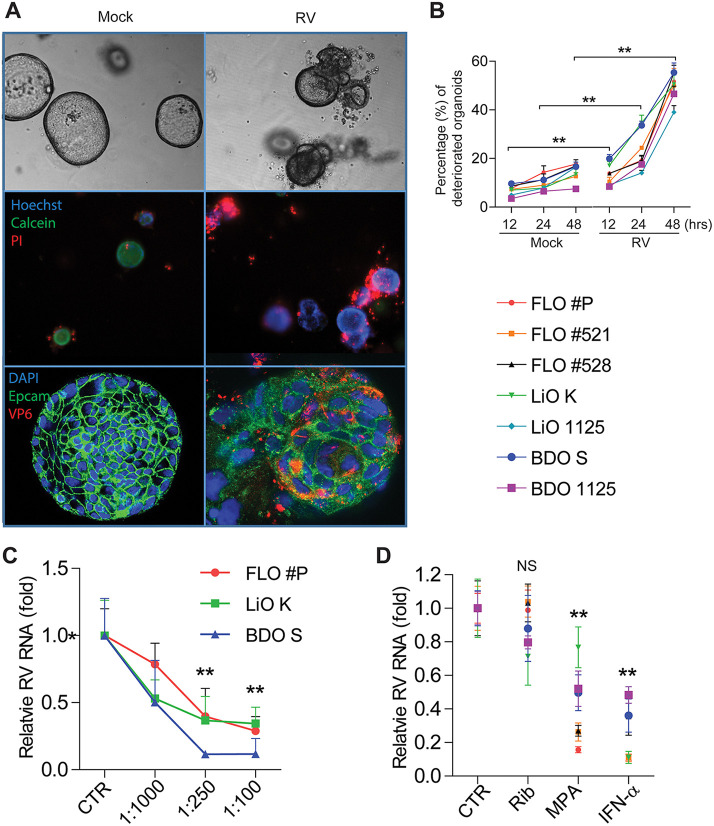
FIG 2.
Cytopathogenesis of rotavirus-infected human biliary organoids and efficacy of antiviral treatment/neutralizing antibody. (A) Organoids from 50 μm to 150 μm in diameter were selected to capture images. Optical microscopy images of infected and uninfected organoids (top). Fluorescence staining of dead cells (PI; red), live cells (calcein; green), and nuclei (Hoechst; blue) (middle). Confocal immunostaining of rotavirus structural protein VP6 (red), epithelial cell adhesion molecule (Epcam; green), and nuclei (blue) (bottom). These are representative images of one FLO batch from the tested seven biliary organoid batches. (B) Quantitative analysis of the percentage of deteriorated organoids with or without rotavirus infection at indicated time points and calculated based on LIVE/DEAD cell staining (A, middle). (C) The inhibitory activities of neutralizing monoclonal antibody HS-1 against rotavirus infection in three representative batches of biliary organoids. (D) The effects of the broad-spectrum antiviral drugs on rotavirus in biliary organoids. Rib, ribavirin; MPA, mycophenolic acid; IFN-α, interferon alpha. All data are presented as means ± SEMs. For each organoid batch, experiments were repeated 3 to 6 times. NS, not significant; **, P < 0.01 by Mann-Whitney test.