Fig. 1.
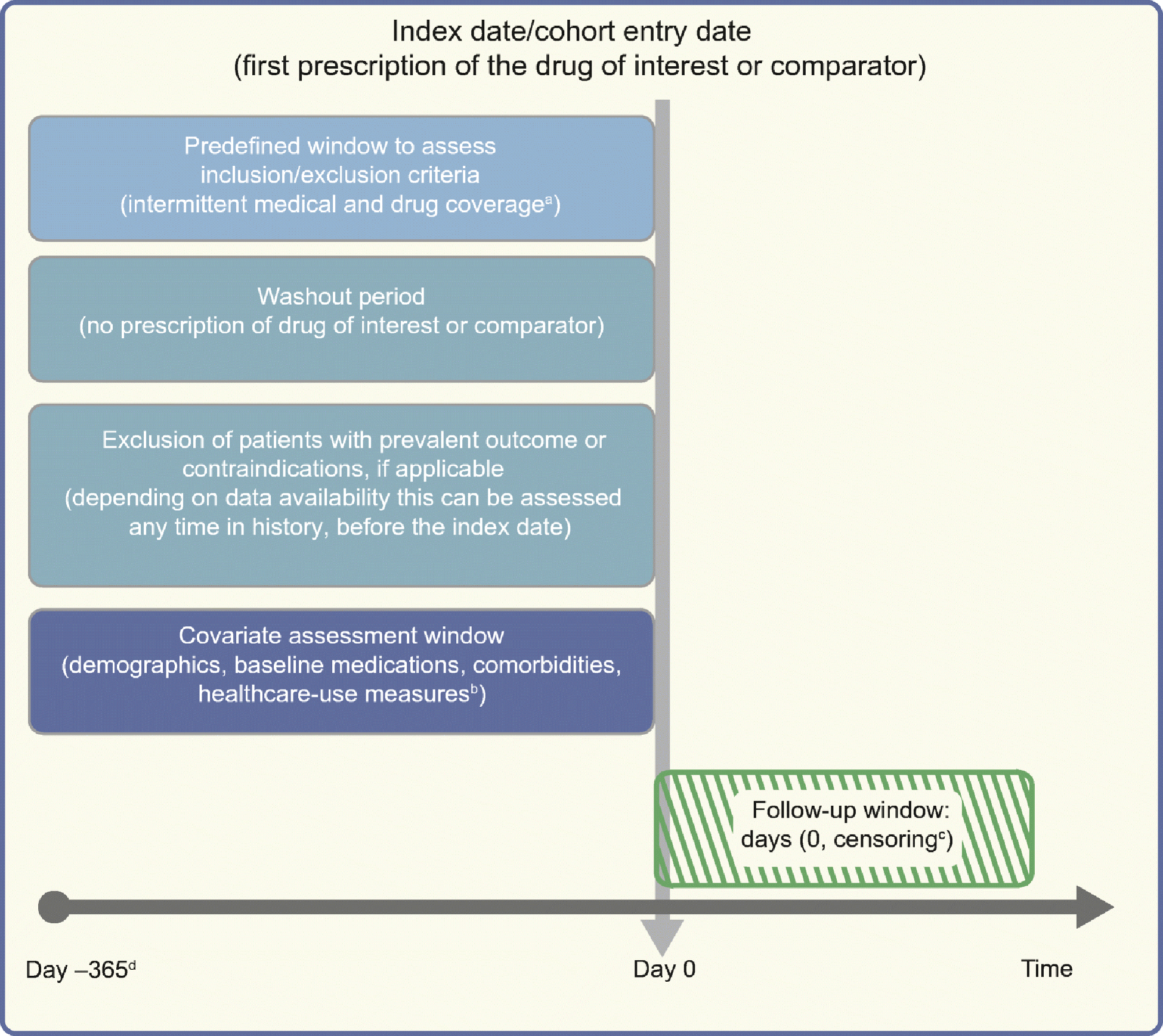
Framework for a cohort study using an administrative claims or electronic medical record database, with methodology from Schneeweiss et al [26], and using templates from www.repeatinitiative.org/projects.html, which are licensed under a Creative Commons Attribution 3.0 Unported License. aTypically, a gap of up to 45 days in medical or pharmacy enrolment is allowed; bcovariates are measured in the 6 month period before entry in to the cohort, and demographics are measured on day zero; cearliest of outcome of interest, switching or discontinuation of study drugs, death, disenrolment, or end of the study period; d365 days pre-index are shown for illustrative purposes; this could be any predefined time before the index date deemed appropriate, and tailored to the study question at hand. This figure is available as part of a downloadable slideset
