Fig. 2.
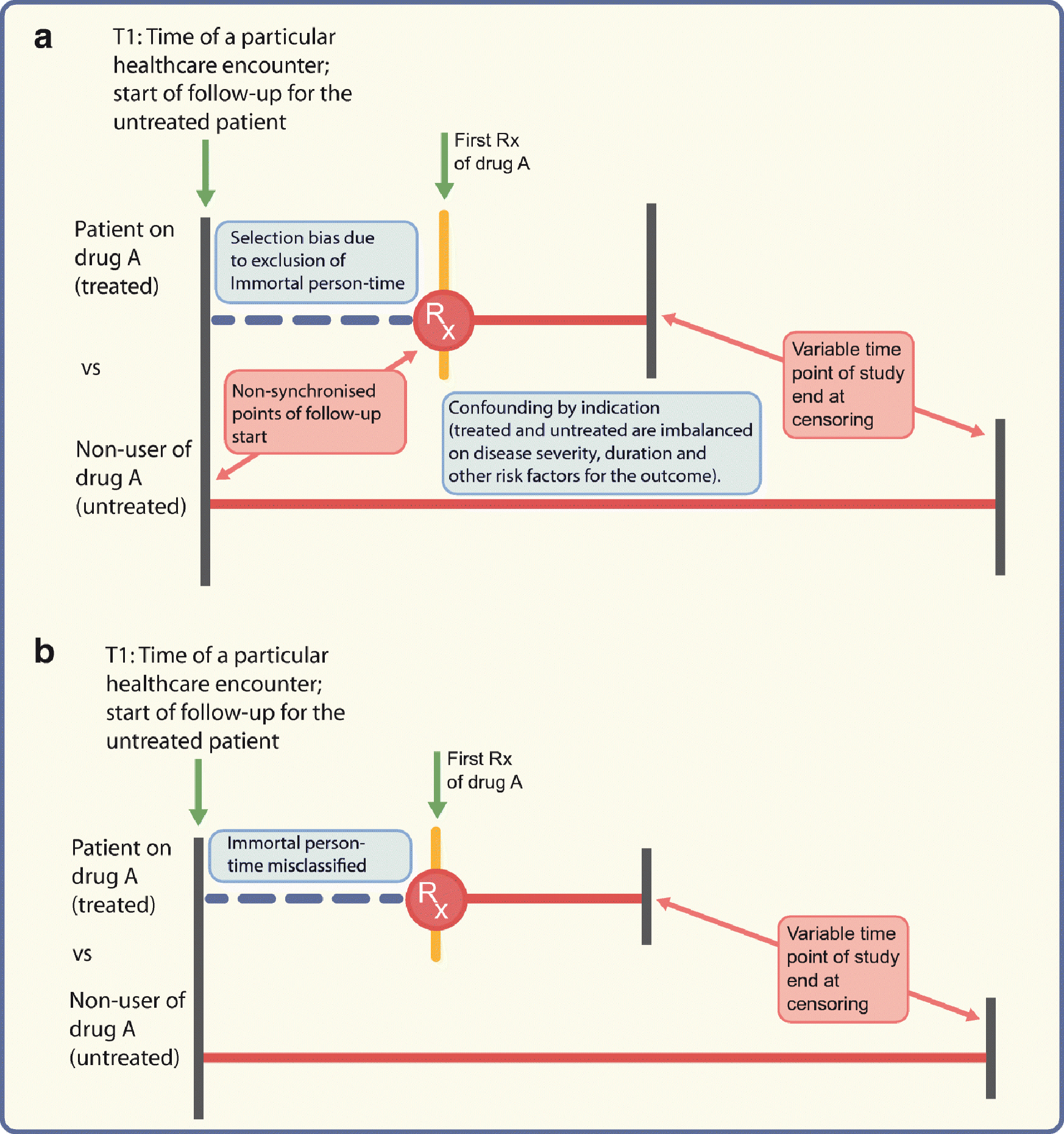
Depiction of problems encountered when comparing treated vs untreated (not using active comparator) patients; drug A could, as an example, be a DPP-4 inhibitor. (a) Different times of follow-up (starting at the initiation date for the treated patients or time of healthcare encounter T1 for the untreated patients) will lead to selection bias if immortal person-time is excluded from the analysis. Confounding by indication may arise from the imbalance between the two groups on ‘indication’. (b) Even if the follow-up for both groups starts from time T1, the time between T1 and drug initiation would be misclassified as ‘time on drug A’ when in reality the patient was not on drug A before the first prescription. Red horizontal lines represent follow-up time. Rx, prescription. This figure is available as part of a downloadable slideset
