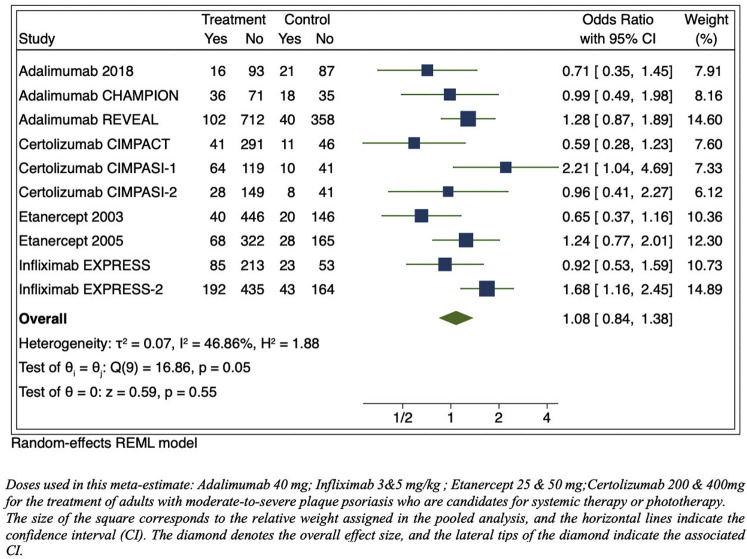
Fig 1.
Meta-estimate of respiratory tract infections from publications of US Food and Drug Administration–approved dosages of phase 3 pivotal trials adverse events tables (includes “upper respiratory tract infections,” “nasopharyngitis,” “rhinitis,” “rhinorrhea,” “pneumonia,” bronchitis,” “sinusitis,” “pharyngitis,” “flu syndrome,” and “cough”). CI, Confidence interval; REML, restricted maximum likelihood.