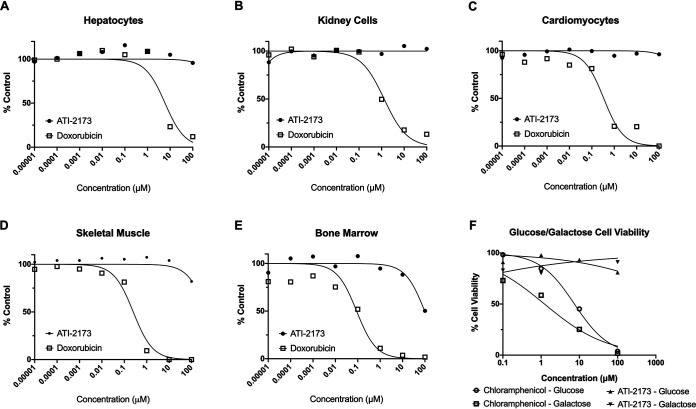
FIG 4.
ATI-2173 is nontoxic at clinically relevant concentrations to hepatocytes, kidney cells, cardiomyocytes, skeletal muscle cells, bone marrow, and mitochondria. ATI-2173 was exposed for 48 to 72 h to hepatocytes (A), kidney cells (B), and cardiomyocytes (C), and was found to exhibit no observable cytotoxicity. Doxorubicin was used as a control. ATI-2173 was exposed for 48 to 72 h to skeletal muscle cells (D) and bone marrow cells (E) and was found to exhibit very mild (D) or mild (E) cytotoxicity at 100 μM doses. (F) ATI-2173 was incubated with HepG2 cells in medium supplemented with either 20 mM glucose or 10 mM galactose. While the chloramphenicol (positive control)-treated cells saw increased cell death in galactose-treated medium compared to glucose-treated medium, cells treated with ATI-2173 saw no appreciable cell death in either glucose or galactose medium.