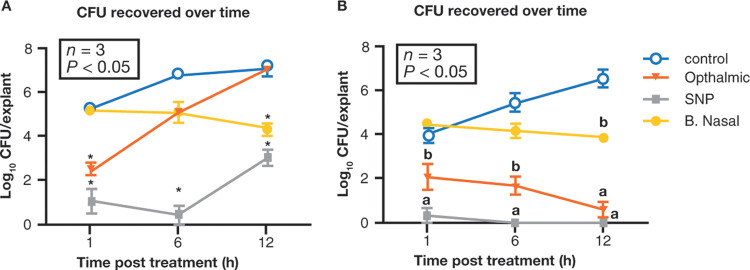
FIG 2.
Efficacy of PVP-I (5% ophthalmic solution or 5% SNP), 2% mupirocin (B. Nasal), or no treatment (control) against MRSA infection in ex vivo models of porcine vaginal mucosa (A) and human skin (B) (adapted from ref. 47). The results are expressed in log10 CFU per explant recovered over time. Values are means ± the standard errors of the means (indicated by error bars). Values that are significantly different (P < 0.05) from untreated controls are indicated by an asterisk in panel A. In panel B, values with a different letter (a, b, or no letter) are significantly different (P < 0.05) from each other, and values with the same letter are not significantly different (P > 0.05) from each other. In panel A, the SNP of 5% PVP-I had significant activity versus the control at all time points, whereas the ophthalmic PVP-I preparation and mupirocin only differed significantly from control at 1 and 12 h, respectively. In panel B, both the SNP and the ophthalmic 5% PVP-I preparations were significantly bactericidal at 1, 6, and 12 h versus the control (P < 0.05), while mupirocin only differed significantly from control at 12 h (P < 0.05). Labels: B. Nasal, Bactroban nasal ointment; MRSA, methicillin-resistant S. aureus; PVP-I, povidone iodine; SNP, skin and nasal preparation.