TABLE 1.
Performance characteristics of nonsilent pncA mutations and proposal for clinical decision making and additional pDST
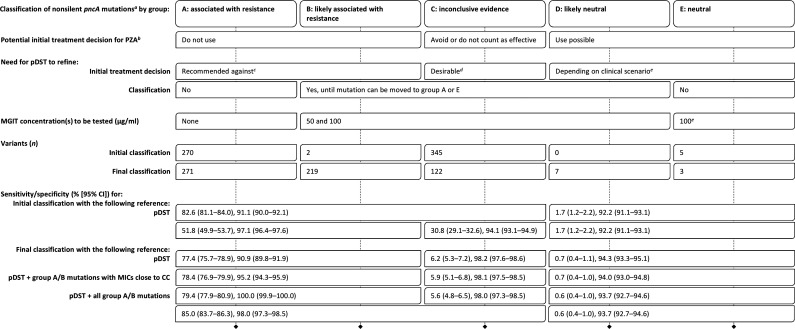
This proposal assumes the use of a high-quality sequencing technology and analysis pipeline with a negligible false-positive rate. All gDST results must be interpreted considering the likely PPV in light of the prevalence of PZA resistance for the strain in question (e.g., otherwise pan-susceptible strains versus MDR strains [29]). If there is a concern regarding the PPV of a pncA result because of the low prevalence of PZA resistance, resequencing would be the appropriate initial test to exclude a random sequencing error.
The regimen may play a role (e.g., some researchers do not consider PZA resistance to be an exclusion criterion for the standardized shorter MDR regimen, despite the current World Health Organization guidelines [30–32]).
Because of the prior evidence that links group A and B mutations with resistance, we recommend not to use PZA for MICs of >50 μg/ml. We even caution against relying on a single MIC of ≤50 μg/ml because of random errors (e.g., laboratory errors). In contrast, if multiple MICs suggest that a group A or B mutation may not be classified correctly, then these findings would have to be reviewed together with the data that underpinned the original classification to revise it if warranted. Additional types of data, such as those from the Wayne assay, may be needed in this context.
For an MIC of ≤50 μg/ml, PZA could be used and counted as effective; for 100 μg/ml, the result is uncertain (i.e., because cutoff errors are possible for pncA mutations), and we recommend to continue to avoid PZA or to use it without counting it as effective; an MIC of >100 μg/ml would be an exclusion criterion for use. Where resources are limited and not all group C mutations can be tested, locally frequent mutations should be prioritized to rapidly identify mutations that are neutral and may, consequently, result in a poor PPV in that setting. If sequencing results were shared in real time, the burden of testing could be shared between countries with low and high incidence and coordinated between laboratories (e.g., different laboratories could be encouraged to test different strains with the same mutation to minimize bias, which we were not able to control for in this review).
To detect resistance due to other mechanisms. It is not clear whether some mutations in other known resistance genes (e.g., panD or rpsA), let alone the yet-unknown mechanisms, also confer MICs close to the CC that would warrant testing 50 and 100 μg/ml for strains with group E mutations (the same consideration applies to strains that harbor no or only synonymous mutations). Until this question is clarified, we propose, if pDST is done at all, to test only the CC to minimize the misclassification of truly susceptible strains as uncertain (i.e., particularly in strains that are otherwise pan-susceptible and are unlikely to be monoresistant to PZA, with the exception of Mycobacterium canettii and most Mycobacterium bovis strains [29, 33–35]).