Fig. 5. The conformational space of AS412 is skewed toward β-hairpin–like conformations in closed HCV E1/E2 states.
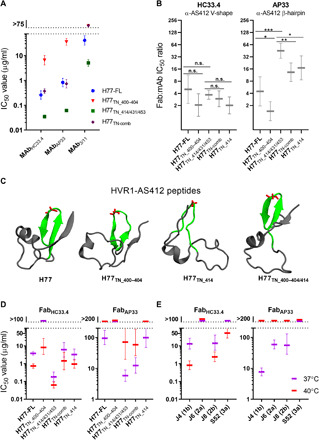
(A) Neutralization using the mAb 3/11, HC33.4, or AP33 of indicated H77 HCVcc recombinants. Data were analyzed as described in Fig. 1. (B) Ratio of Fab:mAb neutralization IC50 values for HC33.4 and AP33 of the indicated H77 recombinants [values calculated from fig. S5 (A and B) dose-response data]. The ratios were compared in two-tailed t tests (GraphPad Prism 8.0.0) with Welch’s correction. Testing was done at the 95% confidence level (*P < 0.05, **P < 0.01, and ***P < 0.001), and the P value was adjusted for multiple testing (*P < 0.025, **P < 0.005, and ***P < 0.0005). (C) Ribbon structures of the most-visited conformations of HVR1-AS412 systems (residues 384 to 426) based on PCA performed on backbone atoms from MD simulations followed by FEL calculations. AS412 is highlighted in green. Residue G418, at the AS412 β-hairpin apex, is highlighted in red. (D and E) Neutralization by HC33.4 and AP33 Fabs against the indicated H77 HCVcc recombinants (D) or HCVcc recombinants with E1/E2 of J4, J6, J8, S52 (E) at 37° or 40°C. Data were analyzed as described in Fig. 1, except using four-parameter dose-response regression (see fig. S6 for dose-response curves).
