Abstract
Objectives
To examine the incidence, prevalence and trends for opioid prescriptions in patients with OA. Furthermore, types of opioids prescribed and long-term prescription rates were examined. Finally, the patient characteristics associated with the prescription of opioids were assessed.
Methods
A population-based cohort study was conducted using the Integrated Primary Care Information database. Incidence and prevalence of opioid prescriptions were calculated for the period 2008–2017. Logistic regression was used to assess which patient characteristics were associated with opioid prescriptions.
Results
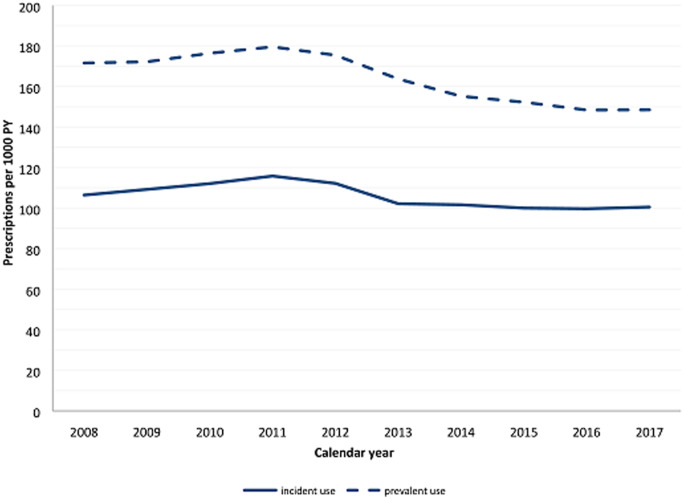
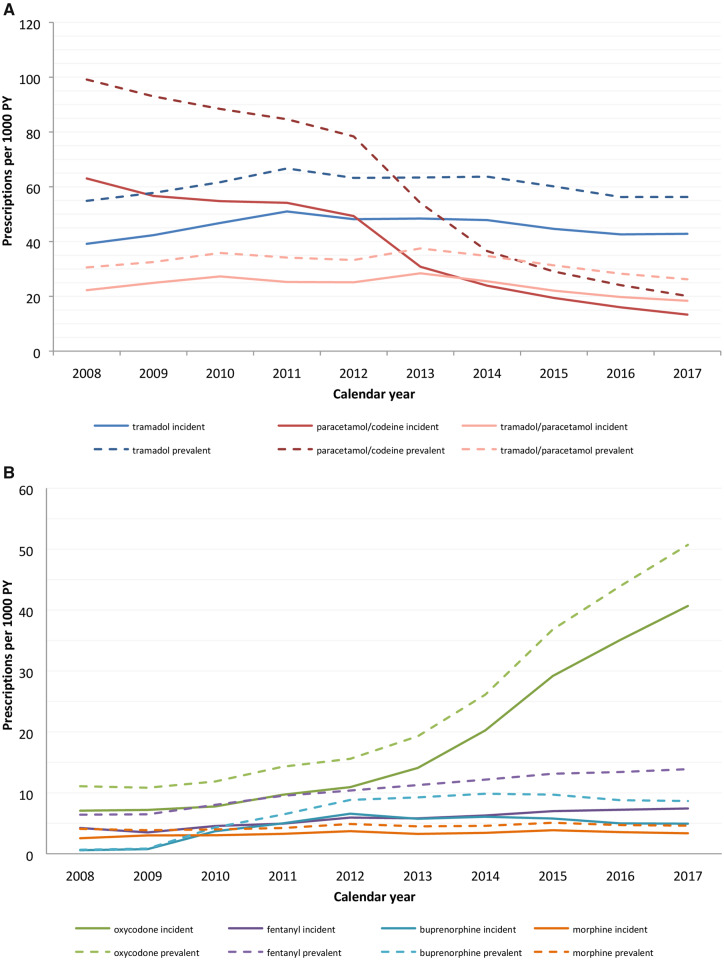
In total, 157 904 OA patients were included. The overall prescription rate remained fairly stable, at around 100 incident and 170 prevalent prescriptions per 1000 person years. However, the incident prescription rate for oxycodone increased from 7.1 to 40.7 per 1000 person years and for fentanyl from 4.2 to 7.4 per 1000 person years. The incident prescription rate for paracetamol/codeine decreased from 63.0 to 13.3 per 1000 person years. Per follow-up year, long-term use was found in 3% of the patients with incident OA. Finally, factors associated with more prescriptions were increasing age, OA in ≥2 joint groups [odds ratio (OR) 1.56; 95% CI: 1.51, 1.65] and the presence of other musculoskeletal disorders (OR 4.91; 95% CI: 4.76, 5.05). Men were less likely to be prescribed opioids (OR 0.78; 95% CI: 0.76, 0.80).
Conclusion
Prescription rates for opioids remained stable, but types of opioids prescribed changed. Oxycodone and fentanyl were increasingly prescribed, while prescriptions of paracetamol/codeine decreased. Since the benefit of opioids for OA pain is questionable and side effects are common, opioids should be prescribed with caution.
Keywords: osteoarthritis, pain, opioids, analgesics
Rheumatology key messages
The overall prescription rates of opioids remained stable over the past decade.
Prescription rates of oxycodone and fentanyl have increased, while prescription rate of paracetamol/codeine has decreased.
Opioids were prescribed for long-term use in 3% of the patients with incident OA.
Introduction
OA is one of the major chronic pain conditions of the musculoskeletal system. Approximately 15% of the population suffers from OA, and the Global Burden of Disease study ranked OA as the 11th biggest contributor to global disability [1, 2]. One of the major complaints of patients with OA is pain. Since the population is ageing and obesity is increasing, it is expected that the disease burden will increase [2].
Treatment options for OA pain consist of exercise therapy, education, weight loss and pain medication, among others. Pain medication is often prescribed in a stepwise approach starting with paracetamol, then topical and oral NSAIDs, followed by opioids if earlier steps do not reduce the pain sufficiently. Nevertheless, the current guidelines for the treatment of OA have a restrictive advice on the use of opioids for OA-related pain [3], or do not recommend the use of opioids [4], since the benefit for OA pain is questionable and treatment is associated with side effects.
Opioids are increasingly being prescribed for chronic non-cancer pain. It has been estimated that around 70% of the prescribed opioids are prescribed for chronic non-cancer pain. Musculoskeletal disorders, including OA, are an important cause of non-cancer pain [5]. The number of opioid prescriptions has risen in the US in particular, more so than in Europe [6]. Opioids do have a potential to cause harm and frequently have side effects, like nausea, constipation and somnolence. In elderly OA patients, the risk of falls and fractures is increased when using opioids [7, 8]. Finally, misuse and addiction are a risk when using opioids [9, 10].
Despite the questionable benefit and the frequency of side effects, the use of opioids in OA patients is high [11, 12] and increasing [13, 14]. Earlier research examined the total use of opioids for OA, but did not focus on which types of opioids were used [11, 13]. Furthermore, knowledge about the long-term use of opioids in OA is scarce.
Therefore, the aim of the current study is to examine the incidence and prevalence of the different types of opioids prescribed in patients with OA and the trends in opioid prescription over the past decade. We examined the long-term prescription rate of opioids in patients with OA and assessed which patient characteristics are associated with opioid prescription.
Methods
Setting
This study was conducted using the Integrated Primary Care Information (IPCI) database. The IPCI database contains the electronic patient records of >1.5 million patients in the Netherlands. The database contains all journal entries by general practitioners (GPs), diagnoses coded according to the International Classification of Primary Care (ICPC) codes, laboratory findings and drug prescriptions [15, 16].
Study cohort
For this study, the use of opioids by patients with OA and aged ≥30 years between 1 January 2008 and 31 December 2017 was examined. Patients who were newly diagnosed with OA in this period (incident OA) as well as patients with a diagnosis of OA in their medical history (prevalent OA) were included in the cohort.
Patients with at least 12 months of valid database history prior to the study entry were included in the cohort. The diagnosis of OA was based on the ICPC codes L84 (spinal OA), L89 (hip OA), L90 (knee OA) and L91 (other peripheral joints affected by OA). Patients younger than 30 were excluded from the cohort (<1% of the patients with a diagnosis of OA), because the use of these ICPC codes in such cases was often a coding error by the GP.
Patients were excluded from the cohort if diagnosed with or having a medical history of malignancy, neuropathic pain disorders or fibromyalgia. Patients were excluded 1 year prior to the first diagnosis of these diseases. If follow-up of the patient was until death, they were excluded from the cohort 1 year before their death. The main reason for exclusion of these patient groups is that there was a probability that opioids were prescribed for reasons other than pain related to OA.
Outcomes
All opioid prescriptions are dispensed by GPs or clinicians in the Netherlands. Opioid prescriptions are identified by Anatomical Therapeutic Chemical (ATC) code. Prescriptions for tramadol (N02AX02), tramadol/paracetamol (N02AJ13), paracetamol/codeine (N02AJ06/N02BE51), oxycodone (N02AA05), fentanyl (N02AB03), morphine (N02AA01) and buprenorphine (N02AE01) were examined since these are commonly prescribed opioids. Hydromorphone, tapentadol, nicomorphine and oxycodone/naloxone were examined, but were rarely prescribed for OA pain (<1.0 prescrition per 1000 person years) and were therefore not considered in the analyses. Tramadol, tramadol/paracetamol and paracetamol/codeine were classified as weak opioids, and oxycodone, fentanyl, morphine and buprenorphine as strong opioids.
The incidence for opioid prescriptions was determined as the total number of new episodes of opioid prescriptions divided by the total number of person years in the cohort per calendar year. A new opioid prescription episode was defined as no prescription in the preceding 6 months. The prevalence of opioid prescriptions was calculated as the total number of patients who had at least one prescription of opioids divided by the total number of person years in the cohort in a calendar year. New episodes of opioid prescriptions within 6 months of a diagnosis of a trauma (e.g. fracture) were excluded in both calculations since it was less likely that those prescriptions were for OA-related pain. Those patients remained in the cohort.
For the patients with incident OA, the number of different types of opioids that were prescribed and the order in which they were prescribed were examined. If patients switched back to an opioid that had been prescribed previously, we did not count this as a new type of opioid. Furthermore, the percentage of patients with incident OA who were prescribed opioids over a long term was calculated. Long-term prescription of opioids was defined as six or more prescriptions during one follow-up year (i.e. in the first year after diagnosis, in the second year after diagnosis, etc.). The median duration of a prescription for opioids is 15 days in the IPCI database and other databases [17]. Therefore, six prescriptions corresponds to an estimated 90 days of opioid use in a year.
Statistical analyses
Descriptive statistics were performed to give the baseline characteristics of the cohort and to calculate prevalence and incidence rates of opioid prescriptions. Univariate logistic regression analyses were conducted to examine whether baseline characteristics were related to opioid prescription, and odds ratios (ORs) and their 95% CI were calculated. Variables were included in the multivariate analysis if P < 0.1 in the univariate analysis. All analyses were performed using SPSS Statistics version 24 (IBM Corp., Armonk, NY, USA).
Study approval
The study was approved by the Board of Directors of the IPCI database.
Results
Study population
In total, 157 904 patients were included in the cohort (Table 1). Of these, 56 713 were newly diagnosed with OA. Of the total patient group, 65.2% were female. Co-morbidities were present in 55.2% of the patient group. The most common type of OA was knee OA (27.8%) and 21.3% of the patients were diagnosed with OA in more than one joint group. At the start of the follow-up period, the mean age was 66.6 (s.d. 12.5) years. During the follow-up period, 65% of the patients visited the GP with another musculoskeletal complaint besides OA (mean follow-up duration was 3 years and 4 months).
Table 1.
Baseline characteristics
| Characteristic | Value (n = 157 904) |
|---|---|
| Age, mean (s.d.), years | 66.6 (12.5) |
| Age category, n (%) | |
| 30–39 | 2587 (1.6) |
| 40–49 | 11 593 (7.3) |
| 50–59 | 31 409 (19.9) |
| 60–69 | 46 924 (29.7) |
| 70–79 | 39 172 (24.8) |
| 80–89 | 22 640 (14.3) |
| ≥90 | 3579 (2.3) |
| Female, n (%) | 102 988 (65.2) |
| Joints affected, n (%) | |
| Back | 16 409 (10.4) |
| Hip | 27 508 (17.4) |
| Knee | 43 919 (27.8) |
| Other joints | 36 410 (23.1) |
| Two or more joints | 33 658 (21.3) |
| Diabetes, n (%) | 22 763 (14.4) |
| Hypertension, n (%) | 63 481 (40.2) |
| Hyperlipidaemia, n (%) | 27 218 (17.2) |
| MI/AP, n (%) | 17 073(10.8) |
| Stroke/TIA, n (%) | 5869 (3.7) |
| PAD, n (%) | 5585 (3.5) |
| UGI/ulcer, n (%) | 5027 (3.2) |
| Heart failure, n (%) | 6306 (4.0) |
| Inflammatory arthritis, n (%) | 7091 (4.5) |
| Other MSD during cohort timea, n (%) | |
| Upper extremity | 36 293 (23.0) |
| Lower extremity | 57 131 (36.2) |
| Back/neck | 47 529 (30.1) |
| Trauma | 18 495 (11.7) |
| Other musculoskeletal | 45 941 (29.1) |
| None | 54 170 (34.3) |
| Renal function, n (%) | |
| eGFR >60 ml/min | 98 928 (62.7) |
| eGFR 30–60 ml/min | 18 298 (11.6) |
| eGFR <30 ml/min | 1 613 (1.0) |
| Missing | 39 065 (24.7) |
Non-specific ICPC diagnoses for complaints of the musculoskeletal system, for example shoulder or hip complaints. The group of back/neck symptoms also includes radiculopathy. AP: angina pectoris; eGFR: estimated glomerular filtration rate; ICPC: International Classification of Primary Care; MI: myocardial infarct; MSD: musculoskeletal disorders; PAD: peripheral arterial disease; TIA: transient ischaemic attack; UGI: upper gastrointestinal.
Trends in incident opioid prescription rate
In the past decade, the overall incident prescription rate for opioids remained fairly stable (Fig. 1). However, the prescription rate for strong opioids was increasing while the prescription rate for weak opioids was decreasing (Figure 2, numbers in Supplementary Table S1, available at Rheumatology online). The incident prescription rate for oxycodone increased from 7.1 per 1000 person years in 2008 to 40.7 per 1000 person years in 2017 and the incident rate for fentanyl increased from 4.2 to 7.4 per 1000 person years. The prescription rate for tramadol/paracetamol decreased from 22.3 to 18.4 per 1000 person years and the prescription rate for paracetamol/codeine decreased between 2008 and 2017 from 63.0 to 13.3 per 1000 person years. In 2013, health insurance companies stopped reimbursing the costs of paracetamol/codeine, which led to the rapid decline since then [18]. The prescription rates for tramadol, morphine and buprenorphine remained stable over the past decade. Buprenorphine was introduced to the market in 2007.
Fig. 1.
Total opioid prescription rate
PY: patient years.
Fig. 2.
Incidence and prevalence of prescribed opioids
(A) Weak opioids; (B) strong opioids. PY: patient years.
Trends in prevalent opioid prescription rate
The trends in prevalent opioid prescription rates showed similar patterns to the incident prescription rates. The overall rate of prescriptions remained stable, while the prescription rate for strong opioids increased and the prescription rate for weak opioids decreased (Figure 2, numbers in Supplementary Table S1, available at Rheumatology online). The major changes were the increase in the prevalent prescription rate for oxycodone from 11.1 to 50.7 per 1000 person years and the decrease in the rate for paracetamol/codeine from 99.1 to 20.1 per 1000 person years.
Long-term prescriptions
In each follow-up year after the diagnosis of OA, 3% of the patients were prescribed opioids over a long term (≥6 prescriptions), representing around a quarter of the patients prescribed any opioid in a given year (Table 2). When comparing strong opioids with weak opioids, the percentage of patients prescribed opioids over a long term was twice as high for patients prescribed strong opioids than for patients prescribed a weak opioid. Furthermore, these percentages did not change with increasing follow-up years.
Table 2.
Chronic users of opioids per follow-up year
| Follow-up time (year) | Total n (% users/% population) | Weak opioids n (% users/% population) | Strong opioids n (% users/% population) |
|---|---|---|---|
| 1 | 1659 (21.5/3.9) | 1025 (16.4/2.5) | 590 (26.4/1.4) |
| 2 | 996 (22.1/3.2) | 601 (16.2/1.9) | 366 (30.0/1.2) |
| 3 | 709 (23.7/3.2) | 401 (16.8/1.8) | 275 (30.8/1.2) |
| 4 | 440 (22.8/3.0) | 234 (15.5/1.6) | 195 (33.0/1.3) |
| 5 | 276 (23.6/3.1) | 142 (15.1/1.6) | 130 (35.2/1.5) |
| 6 | 159 (24.6/3.4) | 82 (16.7/1.7) | 72 (33.2/1.5) |
| 7 | 81 (27.5/3.8) | 34 (15.7/1.6) | 45 (37.8/2.1) |
| 8 | 38 (31.4/4.1) | 20 (21.5/2.1) | 18 (37.5/1.9) |
| 9 | 13 (29.5/4.9) | 7 (22.6/2.6) | 6 (33.3/2.2) |
A chronic user of opioids has ≥6 prescriptions in 1 follow-up year. The number of total chronic users does not equal the sum of the chronic users of weak and strong opioids. Patients can be included in both categories or can be included in the group of total users if the sum of prescriptions for weak and strong opioids is ≥6 while the use of weak opioids only or strong opioids only is not classified as chronic use.
Different types of opioids prescribed
Almost 75% of the patients with incident OA were not prescribed an opioid during the follow-up period (Figure 3A). Of the patients with incident OA, 18.4% were prescribed one type of opioid. A switch to another opioid was made in 8.8% of the patients. Two different types of opioids were prescribed for 5.7% of the patients and 2.1% were prescribed ≥3 types of opioids. Of all patients, 16.4% were prescribed a weak opioid, 5.1% a strong opioid and 4.6% were prescribed both categories. The most common first opioid prescribed to patients was tramadol (39.7%). Oxycodone was prescribed as the first opioid in 17.2% of cases (Figure 3B and Supplementary Table S2, available at Rheumatology online).
Fig. 3.
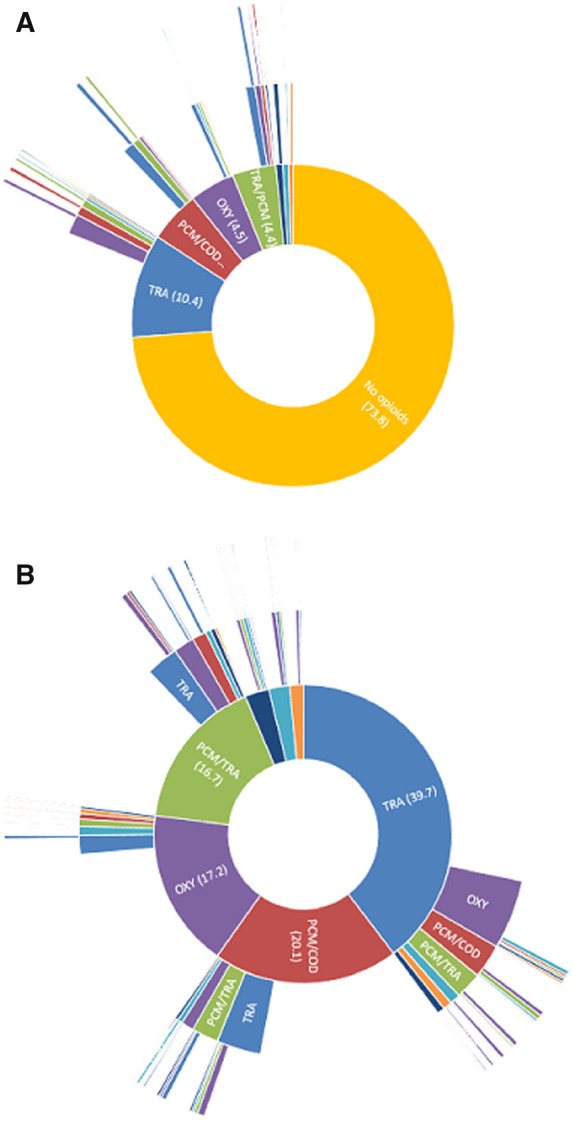
Types of opioids prescribed in patients with incident OA
(A) Types of opioids prescribed in patients with incident OA, including patients not prescribed opioids. (B) Sequence of types of opioids prescribed in patients with incident OA. n = 56 713. The inner circle represents the first opioid prescribed, the second circle the second type of opioid prescribed and the outer circle the third type of opioid prescribed. Percentages are shown in parentheses. BUP (dark blue): buprenorphine; FTY (turquoise): fentanyl; MOP (orange): morphine; OXY (purple): oxycodone; PCM/COD (red): paracetamol/codeine; TRA (blue): tramadol; TRA/PCM (green): tramadol/paracetamol.
Characteristics associated with opioid prescription
In both the univariate and the multivariate regression analyses, the prescription of opioids was associated with a more advanced age at the baseline of the cohort and men were less likely to be prescribed opioids than women (OR 0.78; 95% CI: 0.76, 0.80) (Table 3). Patients with two or more joint groups involved were more likely to be prescribed opioids (OR 1.97; 95% 1.89, 2.05), while patients with OA in other peripheral joints (ICPC code L91) were less likely to be prescribed opioids (OR 0.74; 95% CI: 0.71, 0.78) than patients with back OA. The diagnosis of other musculoskeletal disorders during the follow-up period was associated with the prescription of opioids. Concomitant neck and back problems (OR 3.41; 95% CI: 3.25, 3.57) and the presence of multiple musculoskeletal disorders (OR 4.91; 95% CI: 4.76, 5.05) in particular were associated with a greater likelihood of opioid prescriptions.
Table 3.
Factors associated with opioid prescription
| Factor | Patients prescribed opioid, n (%) | Univariate analysis, OR (95% CI) | Multivariate analysis, OR (95% CI) |
|---|---|---|---|
| Age category | |||
| 30–39 | 495 (19.1) | 1 | |
| 40–49 | 2902 (25.0) | 1.41 (1.27, 1.57) | 1.30 (1.17, 1.46) |
| 50–59 | 8106 (25.8) | 1.47 (1.33, 1.63) | 1.29 (1.16, 1.44) |
| 60–69 | 12 263 (26.1) | 1.50 (1.35, 1.65) | 1.32 (1.19, 1.46) |
| 70–79 | 12 036 (30.7) | 1.88 (1.79, 2.07) | 1.57 (1.41, 1.74) |
| 80–89 | 7305 (32.3) | 2.01 (1.82, 2.23) | 1.73 (1.55, 1.92) |
| >90 | 1036 (28.9) | 1.72 (1.52, 1.95) | 1.72 (1.52, 1.96) |
| Sex | |||
| Female | 31 383 (30.5) | 1 | |
| Male | 12 760 (23.2) | 0.69 (0.67, 0.71) | 0.78 (0.76, 0.80) |
| Joint affected | |||
| Spine | 4273 (26.0) | 1 | |
| Hip | 7172 (26.1) | 1.00 (0.96, 1.05) | 1.05 (1.00, 1.09) |
| Knee | 11 266 (25.7) | 0.98 (0.94, 1.02) | 1.02 (0.98, 1.07) |
| Other joints | 7641 (21.0) | 0.75 (0.72, 0.79) | 0.75 (0.71, 0.78) |
| ≥2 joints | 13 791 (41.0) | 1.97 (1.89, 2.05) | 1.58 (1.51, 1.65) |
| MSD | |||
| None | 7667 (13.4) | 1 | |
| Upper extremity | 1591 (21.6) | 1.78 (1.67, 1.89) | 1.79 (1.69, 1.91) |
| Lower extremity | 3916 (23.9) | 2.03 (1.94, 2.12) | 1.94 (1.86, 2.02) |
| Neck/back | 11 451 (34.4) | 3.38 (3.23, 3.54) | 3.41 (3.25, 3.57) |
| Other MSD | 2542 (24.6) | 2.11 (2.00, 2.22) | 2.09 (1.99, 2.20) |
| ≥2 MSD categories | 24 489 (44.3) | 5.14 (4.99, 5.29) | 4.91 (4.76, 5.05) |
Values in bold are statistically significant. MSD: musculoskeletal disorder.
Discussion
In this study we examined the trends, the long-term prescription and the factors associated with the prescription of opioids in patients with OA. The majority of patients with OA were not prescribed an opioid during the follow-up period. The overall prescription rate of opioids remained fairly stable in the period between 2008 and 2017, with on average 100 incident prescriptions per 1000 person years and 170 prevalent prescriptions per 1000 person years. However, there was a change in the types of opioids that were prescribed. Over time, strong opioids were increasingly prescribed, while prescriptions of weak opioids declined. The prescription rate of oxycodone increased rapidly in this decade from 7.1 to 40.7 incident prescriptions per 1000 person years. The prescription rate for fentanyl also increased from 4.2 to 7.4 incident prescriptions per 1000 person years, while the prescription rates for paracetamol/codeine and tramadol/paracetamol decreased. Weak opioids are still most frequently prescribed for OA-related pain. Around 3% of the patients with incident OA were prescribed opioids over a long term per follow-up year. Finally, factors associated with a greater likelihood of more opioid prescriptions were female sex, increasing age, OA in two or more joint groups, and the presence of other musculoskeletal disorders besides OA.
Most of the studies examining opioid prescriptions in OA patients found an increase in the prescription rate [12–14, 19] while we found a relatively stable prescription rate. One study looking at US insurance data claims also found a relatively stable opioid prescription rate [20]. However, oxycodone is increasingly being prescribed for patients with OA according to the present study. This is in agreement with other studies, which also found increases in the prescription rate of oxycodone for OA [12] and strong opioids for musculoskeletal pain in general [21]. At the same time as the prescription rates for oxycodone and fentanyl increased, the prescription rates for paracetamol/codeine and tramadol/paracetamol decreased. The decline in prescriptions for paracetamol/codeine was caused by the end to reimbursement of the costs by the health insurance companies [18]. This simultaneous increase and decrease could be a coincidence since the increase in strong opioids is also seen in other countries, but it might also be that GPs are prescribing strong opioids instead of weak opioids. Furthermore, the awareness for cardiovascular and gastrointestinal risks of NSAIDs has increased in the past decade and NSAIDs are prescribed less in patients with musculoskeletal disorders [22, 23]. This may also have influenced the prescription rates of (strong) opioids.
The prevalent prescription rate for all opioids was around 170 per 1000 person years in our study. This number is slightly lower than in some other studies [11, 24]. Prescription rates of opioids in general in the Netherlands are lower than or comparable to the prescription rates in the countries covered by these studies (e.g. USA, Sweden and Spain) [6]. Some studies found lower prescription rates for opioids, but these studies did not include tramadol [20] or included only the most commonly prescribed opioids (e.g. tramadol and fentanyl) [13] in the analyses.
Most patients with incident OA were prescribed a weak opioid as their first opioid, which is in line with the current guidelines in the Netherlands [25]. Furthermore, a switch in the type of opioid prescribed was made in 8.8% of the patients with incident OA. A recent study in patients with chronic musculoskeletal pain in the UK also found that weak opioids were most frequently prescribed and that a third of the patients used two or more different opioids. This higher percentage of patients using multiple types of opioids may be because the latter study only included patients with chronic pain. In the Dutch GP guidelines, strong opioids are advised after the prescription of weak opioids [25]. However, almost a quarter of the patients with incident OA were directly prescribed a strong opioid.
Around 3% of the patients with incident OA were prescribed opioids over a long term (≥6 prescriptions per follow-up year). No previous data on long-term use specific to OA is available to our knowledge, but this percentage is comparable to long-term use in musculoskeletal disorders in general [26, 27]. We did not find an increase in long-term use with increasing follow-up time. Analyses per calendar year showed similar results (data not shown). The available evidence on whether long-term use of opioids for musculoskeletal disorders is increasing gives conflicting results [26, 27].
Factors associated with opioid prescription were increasing age, female sex, the presence of other musculoskeletal disorders and multiple joint groups affected by OA. These characteristics are also found in other studies [14, 28, 29]. The higher prescription rates in older patients is concerning since use of opioids in this group is associated with more severe side effects like falls and fractures [7, 8].
The high rates and long-term use of opioids for OA pain are concerning since the absolute number of patients is high. As mentioned earlier, guidelines have a restrictive advice on the use of opioids [3, 4]. Furthermore, a recent study showed that treatment with opioids for patients with OA or for patients with low back pain did not result in better pain relief than treatment with non-opioid analgesics (e.g. paracetamol and NSAIDs) [30]. In addition, long-term use is associated with dose-dependent adverse events, like trauma and addiction [7, 31], and does not lead to an improved quality of life [32].
A strength of the current study is that it was conducted using a database that is a representative sample of the Dutch population. That is because in the Netherlands most opioids are prescribed by the GP. Initial data show that around 70% of the first prescriptions and around 90% of the repeat prescriptions of opioids are prescribed by the GP [33].
There are several limitations to this study. Firstly, patients were only included in the cohort if diagnosed with OA. Since GPs vary in how strictly they apply the ICPC codes, this may have led to an overestimation or underestimation of patients with OA. Studies using UK primary healthcare databases showed that the positive predictive value of hip OA codes is sufficiently high [34], but that OA is probably under-recorded [35]. Furthermore, in the medical records the indication of the opioid prescription could not be directly linked to a diagnosis. We excluded patients with malignancy, neuropathic pain disorders or fibromyalgia and patients in the year before death. Nevertheless we cannot rule out the possibility that some opioid prescriptions will be for reasons other than OA-related pain. We did not exclude all other musculoskeletal comorbidities for which opioids can be prescribed. These are frequently present in OA patients [36] and we found that the presence of musculoskeletal comorbidities was positively associated with opioid prescriptions.
To conclude, the overall prescription rate of opioids remained fairly stable in the past decade. However, the prescription rate for oxycodone increased rapidly and the prescription rate for fentanyl almost doubled, while the prescription rates for paracetamol/codeine and tramadol/paracetamol decreased. Weak opioids are more commonly prescribed for OA pain than strong opioids. The increase in prescriptions of strong opioids is concerning since clinically relevant effectiveness is questionable for OA pain and serious side effects are common. Therefore, opioids should be prescribed with caution, especially for long-term use, and alternative strategies for reducing pain and for reducing long-term opioid use should be considered.
Supplementary Material
Acknowledgements
J.J.vdD., D.S., M.dW., P.J.E.B., J.vdL., S.M.A.B-Z. participated in the design of the study. J.J.vdD. and M.dW. conducted statistical analysis. J.J.vdD., D.S., M.dW., P.J.E.B., J.vdL., S.M.A.B-Z. were involved in the writing, editing and approval of the final manuscript.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for profit sectors to carry out the work described in this manuscript.
Disclosure statement: S.M.A.B-Z. reports grants from The Netherlands Organisation for Health Research and Development, the Dutch Arthritis Association, the European Commission 7th Framework Programme, the European Institute of Innovation and Technology Health, and the Foundation for Research in Rheumatology, personal fees from Infirst healthcare, personal fees from Osteoarthritis Research Society International, outside the submitted work. J.vdL. reports grants from The Netherlands Organisation for Health Research and Development, the European Commission Framework Programme, and a number of pharmaceutical companies, outside the submitted work. The other authors declare no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Johnson VL, Hunter DJ.. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol 2014;28:5–15. [DOI] [PubMed] [Google Scholar]
- 2. Cross M, Smith E, Hoy D. et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73:1323–30. [DOI] [PubMed] [Google Scholar]
- 3. Zhang W, Doherty M, Arden N. et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2005;64:669–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McAlindon TE, Bannuru RR, Sullivan MC. et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363–88. [DOI] [PubMed] [Google Scholar]
- 5. Birke H, Kurita GP, Sjogren P. et al. Chronic non-cancer pain and the epidemic prescription of opioids in the Danish population: trends from 2000 to 2013. Acta Anaesthesiol Scand 2016;60:623–33. [DOI] [PubMed] [Google Scholar]
- 6. Helmerhorst GT, Teunis T, Janssen SJ, Ring D.. An epidemic of the use, misuse and overdose of opioids and deaths due to overdose, in the United States and Canada: is Europe next? Bone Joint J 2017;99-B:856–64. [DOI] [PubMed] [Google Scholar]
- 7. Lo-Ciganic WH, Floden L, Lee JK. et al. Analgesic use and risk of recurrent falls in participants with or at risk of knee osteoarthritis: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2017;25:1390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rolita L, Spegman A, Tang X, Cronstein BN.. Greater number of narcotic analgesic prescriptions for osteoarthritis is associated with falls and fractures in elderly adults. J Am Geriatr Soc 2013;61:335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noble M, Treadwell JR, Tregear SJ. et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev 2010;(1):CD006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheatle MD. Prescription opioid misuse, abuse, morbidity, and mortality: balancing effective pain management and safety. Pain Med 2015;16(Suppl 1):S3–8. [DOI] [PubMed] [Google Scholar]
- 11. Thorlund JB, Turkiewicz A, Prieto-Alhambra D, Englund M.. Opioid use in knee or hip osteoarthritis: a region-wide population-based cohort study. Osteoarthritis Cartilage 2019;27:871–7. [DOI] [PubMed] [Google Scholar]
- 12. Ackerman IN, Zomer E, Gilmartin-Thomas JF, Liew D.. Forecasting the future burden of opioids for osteoarthritis. Osteoarthritis Cartilage 2018;26:350–5. [DOI] [PubMed] [Google Scholar]
- 13. Wilson N, Sanchez-Riera L, Morros R. et al. Drug utilization in patients with OA: a population-based study. Rheumatology (Oxford) 2015;54:860–7. [DOI] [PubMed] [Google Scholar]
- 14. Wright EA, Katz JN, Abrams S, Solomon DH, Losina E.. Trends in prescription of opioids from 2003-2009 in persons with knee osteoarthritis. Arthritis Care Res (Hoboken) 2014;66:1489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Lei J, Duisterhout JS, Westerhof HP. et al. The introduction of computer-based patient records in The Netherlands. Ann Intern Med 1993;119:1036–41. [DOI] [PubMed] [Google Scholar]
- 16. Vlug AE, van der Lei J, Mosseveld BM. et al. Postmarketing surveillance based on electronic patient records: the IPCI project. Methods Inf Med 1999;38:339–44. [PubMed] [Google Scholar]
- 17. Weesie Y, van Dijk L, Flinterman L, Hek K. Voorschrijven van opioïden in de huisartsenpraktijk. 2016. https://www.nivel.nl/sites/default/files/bestanden/Rapport_voorschrijven_opioiden.pdf (16 April 2019, date last accessed).
- 18.Zorginstituut Nederland. Uitstroomadvies Paracetamol-Codeïne. 2012. https://www.zorginstituutnederland.nl/publicaties/adviezen/2012/10/15/uitstroomadvies-paracetamol-codeine (16 April 2019, date last accessed).
- 19. Yu D, Jordan KP, Bedson J. et al. Population trends in the incidence and initial management of osteoarthritis: age-period-cohort analysis of the Clinical Practice Research Datalink, 1992-2013. Rheumatology (Oxford) 2017;56:1902–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeMik DE, Bedard NA, Dowdle SB. et al. Are we still prescribing opioids for osteoarthritis? J Arthroplasty 2017;32:3578–82.e1. [DOI] [PubMed] [Google Scholar]
- 21. Foy R, Leaman B, McCrorie C. et al. Prescribed opioids in primary care: cross-sectional and longitudinal analyses of influence of patient and practice characteristics. BMJ Open 2016;6:e010276.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koffeman AR, Valkhoff VE, Jong GW. et al. Ischaemic cardiovascular risk and prescription of non-steroidal anti-inflammatory drugs for musculoskeletal complaints. Scand J Prim Health Care 2014;32:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmidt M, Sorensen HT, Pedersen L.. Diclofenac use and cardiovascular risks: series of nationwide cohort studies. BMJ 2018;362:k3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee SW, Patel J, Kim SY. et al. Use of opioid analgesics in patients with chronic low back pain and knee osteoarthritis. Am J Phys Med Rehabil 2019;98:e97–8. [DOI] [PubMed] [Google Scholar]
- 25.NHG. NHG Standaard Pijn (M106). 2018. https://www.nhg.org/standaarden/volledig/nhg-standaard-pijn (15 September 2019, date last accessed).
- 26. Boudreau D, Von Korff M, Rutter CM. et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009;18:1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thielke SM, Simoni-Wastila L, Edlund MJ. et al. Age and sex trends in long-term opioid use in two large American health systems between 2000 and 2005. Pain Med 2010;11:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campbell CI, Weisner C, Leresche L. et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health 2010;100:2541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Power JD, Perruccio AV, Gandhi R. et al. Factors associated with opioid use in pre-surgical knee, hip and spine osteoarthritis patients. Arthritis Care Res (Hoboken) 2019;71:1178. [DOI] [PubMed] [Google Scholar]
- 30. Krebs EE, Gravely A, Nugent S. et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018;319:872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bedson J, Chen Y, Ashworth J. et al. Risk of adverse events in patients prescribed long-term opioids: a cohort study in the UK Clinical Practice Research Datalink. Eur J Pain 2019;23:908–22. [DOI] [PubMed] [Google Scholar]
- 32. Hayes CJ, Payakachat N, Li C.. Evaluation of opioid use among patients with back disorders and arthritis. Qual Life Res 2018;27:3021–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coolen van Brakel R, van Rijn van Alkemade E. Verslag Rondetafelconferentie VWS gebruik Opioïden. Utrecht: Instituut Verantwoord Medicijngebruik, 2018. https://www.medicijngebruik.nl/filedispenser/19E906CC-82D8-429F-B53D-82897A860693 (15 September 2019, date last accessed). [Google Scholar]
- 34. Ferguson RJ, Prieto-Alhambra D, Walker C. et al. Validation of hip osteoarthritis diagnosis recording in the UK Clinical Practice Research Datalink. Pharmacoepidemiol Drug Saf 2019;28:187–93. [DOI] [PubMed] [Google Scholar]
- 35. Yu D, Jordan KP, Peat G.. Underrecording of osteoarthritis in United Kingdom primary care electronic health record data. Clin Epidemiol 2018;10:1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Picavet HS, Hazes JM.. Prevalence of self reported musculoskeletal diseases is high. Ann Rheum Dis 2003;62:644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.