Abstract
Objective
To evaluate drug survival with monotherapy compared with combination therapy with MTX in RA older adults.
Methods
Patients from the British Society for Rheumatology Biologics Register, a prospective observational cohort, who were biologic naïve and commencing their first TNF inhibitors (TNFi) were included. The cohort was stratified according to age: <75 and ≥75. Cox-proportional hazards models compared the risk of TNFi discontinuation from (i) any-cause, (ii) inefficacy and (iii) adverse events, between patients prescribed TNFi-monotherapy compared with TNFi MTX combination.
Results
The analysis included 15 700 patients. Ninety-five percent were <75 years old. Comorbidity burden and disease activity were higher in the ≥75 cohort. Fifty-two percent of patients discontinued TNFi therapy during the follow-up period. Persistence with therapy was higher in the <75 cohort. Patients receiving TNFi monotherapy were more likely to discontinue compared with patients receiving concomitant MTX [hazard rate 1.12 (1.06–1.18) P <0.001]. This finding only held true in patients <75 [hazard rate (HR) 1.11 (1.05–1.17) vs ≥75 [HR 1.13 (0.90–1.41)]. Examining TNFi discontinuation by cause revealed patients ≥75 receiving TNFi monotherapy were less likely to discontinue TNFi due to inefficacy [HR 0.66 (0.43–0.99) P=0.04] and more likely to discontinue therapy from adverse events [HR 1.41(1.02–1.96) P =0.04]. These results were supported by the multivariate adjustment in complete case and imputed analyses.
Conclusion
TNFi monotherapy is associated with increased treatment failure. In older adults, the disadvantage of TNFi monotherapy on drug survival is no longer seen. Patients ≥75 have fewer discontinuations due to inefficacy than adverse events compared with younger patients. This likely reflects greater disposition to toxicity but perhaps also a decline in immunogenicity associated with immunosenescence.
Keywords: rheumatoid arthritis, biologics, anti-TNF therapy, methotrexate, epidemiology
Rheumatology key messages
TNFi monotherapy is associated with increased treatment failure in RA.
In RA patients ≥75, the disadvantage of TNFi monotherapy on drug survival is no longer seen.
Older RA patients have fewer discontinuations due to inefficacy compared with younger patients.
Introduction
In the management of RA, MTX continues to serve as the ‘anchor drug’, demonstrating efficacy as a first-line therapy and is established as the standard of care worldwide [1]. Biologics are routinely used in patients who have failed treatment with MTX and/or other conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). Current national UK guidelines advocate administering biologics in combination with MTX therapy for those patients with an inadequate response to csDMARDs alone.
Randomized controlled trial data consistently demonstrate superior efficacy in controlling disease activity with TNF blockade in combination with MTX over TNF inhibitors (TNFi) monotherapy [2–7]. Longer-term observational data from national registries allow the examination of treatment continuation rates (drug survival). Drug survival is influenced by various factors including lack or loss of clinical efficacy, adverse events and poor adherence. Despite a good initial response to a TNF inhibitor, efficacy can wane over time. Secondary failure may result from the formation of antidrug antibodies generated as a consequence of an immune response to the protein base agent, potentially neutralizing its therapeutic effect. Concomitant immunosuppression with MTX has a synergistic advantage. MTX increases TNFi concentrations via the suppression of antidrug antibodies, prolonging TNFi drug survival [8].
Registry data suggest superior drug survival with TNFi MTX combination compared with TNFi monotherapy [9–11]. A systematic review of published data from European and non-European registries reported that TNFi/csDMARD combinations reduced the risk of discontinuations from lack of efficacy [12]. Individual registries also describe superior survival rates with TNFi/csDMARD combinations, driven by fewer terminations from adverse events [13].
Adults aged over 65 years old are under-represented in RA clinical trials and data mainly originates from post hoc analyses. Whilst the efficacy and safety of TNF blockade in patients over 65 years has been examined in observational studies, the results are conflicting [14–19]. Some report reduced efficacy of TNFi in the elderly [14, 18] whilst other studies have not demonstrated an association with age and treatment response [15, 17] or rates of TNFi discontinuation [16]. The reasons for TNFi discontinuation may differ depending on age, with older patients discontinuing more frequently as a result of adverse events and younger patients as a result of inefficacy [17, 20].
Older age may associate with a reduction in the immunogenicity of biologic therapies. The aging immune system undergoes a gradual process of decline, termed immunosenescence. This affects both the innate and adaptive arms of the immune response. Key feature includes the suppression of phagocytosis by neutrophils and macrophages, altered cytokine production and a decrease in number and function of T and B lymphocytes and NK cells [21–26]. T-cell diversity is maintained in patients up to 65 years of age, despite thymic output ceasing by ∼50. After this, there is a rapid loss of clonal heterogeneity in individuals aged 75–80 years, with the T-cell repertoire diversity a mere 1% that of a younger cohort [27]. With increasing age there are important changes in antibody diversity with a decline in the ability to produce specific antibodies [24]. It is plausible that the production of antidrug antibodies that neutralize the effect of TNF inhibitors is less robust in elderly adults, reducing the risk of secondary failure and eliminating the need for concomitant immunosuppression.
The primary objective of this study was to investigate drug survival rates with TNFi monotherapy compared with combination therapy with MTX in older adults. We hypothesise that TNFi drug survival is different in these patients and the use of combination therapy might not prove as advantageous in older adults as it is in the younger cohort.
Methods
Patient population
Patients in this analysis were participants in the British Society of Rheumatology Biologics Register for RA (BSRBR-RA), a national prospective observational cohort study established in 2001 to monitor long-term safety of biological therapy. The BSRBR-RA methodology has been described previously [28]. Ethical approval was granted in 2000 [North West MREC (Multicentre Research Ethics Committee), reference 00/8/053]. Data uploaded to the BSRBR-RA by June 2016 were included in this analysis. All patients with RA who were biologic naïve and commencing their first TNF inhibitors (infliximab, etanercept, adalimumab and certolizumab) were eligible for inclusion in the analysis. The initial BSRBR-RA biologic cohorts in 2001 were for etanercept and infliximab users. Adalimumab and certolizumab-pegol cohorts were recruited later. A golimumab cohort has not been recruited. We chose a cut-off in age at 75 years a priori for the primary analysis for pragmatic reasons. Previously analyses have used an age of 65, although this is probably too young to anticipate a difference attributable to immunosenescence. Due to diminishing sample sizes it would have been inappropriate to select a sample any higher than 75 years. Our exploratory analyses have considered other age cut-off points (Supplementary Table S1, available at Rheumatology online).
Baseline data
At registration, baseline data included demographics, comorbidity, smoking status, RA disease duration, RA disease activity (28-joint count Disease Activity Score), HAQ and csDMARD and corticosteroid exposure. Comorbidities were obtained from the patient’s medical records, using a pre-specified list of coexisting conditions. Comorbidity burden was scored using the Rheumatic Disease Comorbidity Index (RDCI), composed of 11 weighted past or present comorbid conditions. The RDCI performs well in predicting RA specific outcomes including disability, medical costs, hospitalisation and death [29–31].
Follow-up
Follow-up data were collected every 6 months for the first 3 years by questionnaires sent to patients and their supervising rheumatology teams, and annually thereafter by questionnaires sent to the supervising rheumatology team only. Data on adverse events were captured from clinician questionnaires, from 6-monthly patient diaries detailing new hospital admissions, and by linkage to NHS Digital, which provides mortality data. NHS Digital has near complete capture of mortality data in the UK as all deaths (irrespective of where the death occurs) are centrally registered.
Outcome
The primary outcome was persistence with first TNFi therapy, which was defined as the duration of time the patients continued to receive TNF blockade. Individuals were considered ‘at risk’ from treatment start for 5 years, or until treatment stop date, date of the last follow-up or date of death, whichever came first. Temporary stops of <90 days, after which the patient restarted the same anti-TNF therapy were counted as continuous use of the drug. Secondary outcomes included reason for TNF discontinuation separated according to inefficacy and adverse events.
Statistical analysis
The cohort was divided according to age at registration: <75 and ≥75 years. Baseline characteristics were tabulated and tested for statistically significant imbalance using χ2, Mann–Whitney or t-tests, as appropriate. Kaplan–Meier survival curves were used to describe the persistence with anti-TNF therapy. The incidence rate (IR) of treatment discontinuation was calculated per 100 patient-years with 95% CI. Cox proportional hazards models were used to compare the risk of TNFi discontinuation between patients prescribed TNFi monotherapy compared with those receiving TNFi MTX combination (the reference group). Three models were developed, evaluating treatment discontinuation: (i) any cause, (ii) inefficacy and (iii) adverse events. For the separate inefficacy and adverse event analyses, a competing risk survival model was used following the Fine & Gray method allowing for accurate estimates of cumulative incidence [32]. Multivariable adjustment was made for the following baseline covariates: age, sex, disease duration, DAS28, HAQ, RDCI, smoking status and steroid exposure.
Baseline missing data were addressed using multiple imputation, with multivariate sequential imputation using chained equations for 20 imputations (Supplementary Table S2, available at Rheumatology online). The HAQ-DI was analysed as a continuous variable. We did not have access to item-level data for the HAQ-DI to Rasch transform it. We used predictive mean matching approach in the imputation model to account for this.
To address confounding by indication, a sensitivity analysis was performed using a propensity score (PS) model employing inverse probability of treatment weights for patients receiving TNFi monotherapy compared with those receiving TNFi MTX combination. The PS model included the following baseline covariates: age, sex, disease duration, DAS28, HAQ, RDCI, smoking status and steroid exposure (Supplementary Table S3, available at Rheumatology online).
Further analyses compared TNFi discontinuation in patients prescribed TNFi with other csDMARDs combinations. All analyses were undertaken using Stata 15 (StataCorp., College Station, TX, USA).
Results
Patient characteristics
Of 23 411 subjects registered in the BSRBR-RA, 15 700 were biologic naïve and commencing their first TNF inhibitor. Ninety-five percent of the cohort were younger than 75 years old. Overall mean age was 55 (s.d. 12.9), with a median disease duration of 10 years [interquartile range (IQR) 5–18]. Baseline mean DAS-28 was 6.42 (s.d. 1.06), reflective of a UK biologic initiation cohort. Baseline characteristics are in Table 1.
Table 1.
Baseline characteristics by age group
| <75 years old | ≥75 years old | Stat. imbalance | |
|---|---|---|---|
| Total cohort, n (%) | 14 932 (95.1) | 768 (4.9) | |
| Age, yrs, | 55 (46–63) | 77 (76–80) | |
| Female sex, n (%) | 10 788 (72.3) | 627 (81.6) | <0.001b |
| Smoking status, n (%) | |||
| Current | 2648 (22.3) | 43 (7.0) | <0.001b |
| Ever | 7597 (61.6) | 393 (60.3) | 0.53b |
| Comorbidity (RDCI score ≥1), n (%) | 8303 (55.6) | 551 (71.7) | <0.001b |
| Cardiac (MI, stroke, angina) | 968 (6.5) | 133 (17.3) | <0.001b |
| Respiratory (asthma, COPD) | 2080 (13.9) | 129 (16.9) | <0.03b |
| Seropositive (RF), n (%) | 8437 (58.7) | 485 (64.3) | <0.002b |
| Disease duration, yrs | 10 (5–18) | 14 (7–23) | <0.0001a |
| Number of previous csDMARDs | 3 (2–5) | 3 (2–5) | 0.33a |
| TNFi, n (%) | 0.82b | ||
| Infliximab | 3955 (26.5) | 209 (27.2) | |
| Etanercept | 5374 (36.0) | 265 (34.5) | |
| Adalimumab | 4744 (31.8) | 246 (32.0) | |
| Certolizumab | 859 (5.8) | 48 (6.3) | |
| TNFi monotherapy, n (%) | 3642 (24.4) | 268 (34.9) | <0.001b |
| TNFi/csDMARDs combination | |||
| Methotrexate | 5776 (38.7) | 252 (33.8) | |
| Sulfasalazine | 430 (2.9) | 21 (2.7) | |
| Leflunomide | 667 (4.5) | 43 (5.6) | |
| Two csDMARDs | 2930 (19.6) | 111 (14.5) | |
| Three csDMARDs | 781 (5.2) | 30 (3.9) | |
| Other combination | 706 (4.7) | 43 (5.6) | |
| Prednisolone, n (%) | 5867 (39.3) | 401 (52.2) | <0.001b |
| DAS28-ESR, mean (s.d.) | 6.42 (1.1) | 6.52 (1.0) | 0.01a |
| SJC28, mean (s.d.) | 10.7 (6.2) | 10.6 (6.0) | 0.84a |
| TJC28, mean (s.d.) | 15.2 (7.5) | 15.1 (7.9) | 0.67a |
| Global VAS | 75 (62-87) | 75 (60–87) | 0.20a |
| ESR | 38 (21–61) | 43 (26–68) | <0.0001a |
| CRP mg/l | 26 (11–56) | 29 (13–60) | 0.12a |
| HAQ, median (IQR) | 2.125 (1.625-2.375) | 2.25 (2–2.625) | <0.0001 |
All values are gives as median (IQR), unless otherwise specified by n (%) or mean (s.d.).
Statistical imbalance tested by kwallis.
Statistical imbalance tested by χ2.
TNFi: TNF inhibitor; RDCI: Rheumatic Disease Comorbidity Index; DAS28: Disease Activity Score 28 Joints; SJC28: 28 swollen joint count; TJC28: 28 tender joint count; Global VAS: visual analogue scale for patient’s global assessment.
Patients 75 years and older
As expected, the ≥75 cohort demonstrated greater comorbidity burden compared with the younger cohort (RDCI score ≥1 in 72% vs 56%, P < 0.001), with a higher prevalence of both cardiac and respiratory disease. RA disease activity measured by DAS28-ESR was higher in the ≥75 cohort (mean DAS28 6.52 vs 6.42, P = 0.009). This was driven by a higher ESR [median 43 (IQR 26–68) vs 38 (21–61), P < 0.0001] with no significant difference in the number of tender and swollen joints or global visual analogue scale (VAS) between the two age groups. A greater proportion of the ≥75 cohort were prescribed prednisolone (52% vs 39%, P < 0.001); however, there was no difference in the number of previous csDMARDs or choice of TNFi agents. Older patients were more likely to be prescribed TNFi monotherapy over combination with csDMARDs (35% vs 24%, P < 0.0001).
Seventy-five percent of patients were prescribed TNFi in combination with csDMARDs, rather than as monotherapy. There were several key differences comparing patients on TNFi monotherapy to combination therapy; patients on TNFi monotherapy demonstrated greater comorbidity burden, elevated markers of RA disease activity and disability, and a higher number of previous failed csDMARDs and concurrent prednisolone exposure (Supplementary Table S4, available at Rheumatology online).
Persistence of TNF blockade
Fifty-two percent of the cohort (n = 8206) discontinued their first TNFi therapy during the follow-up period. With 44 642 person-years follow-up, the overall incidence of discontinuation was 18.4 (95% CI: 18.0, 18.8) per 100 patient years. Major reasons for discontinuation were adverse event (40%) and inefficacy (41%).
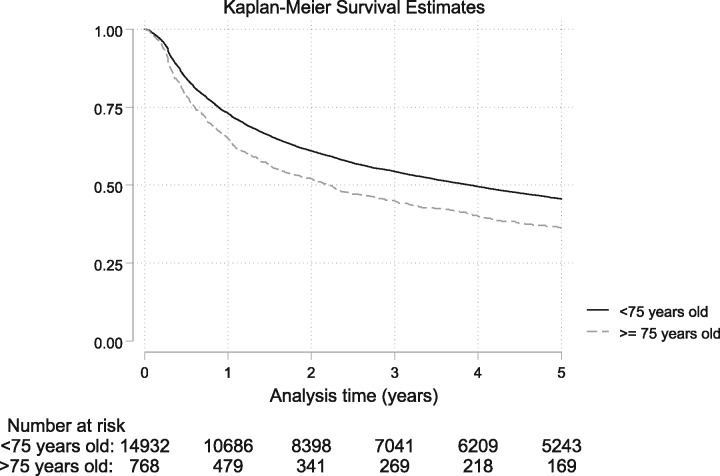
Persistence with TNFi therapy was higher in the younger cohort (Fig. 1). The crude IRs per 100 patient years for TNFi discontinuation were higher in the ≥75 compared with <75 age group; all cause: IR 25.5 (95% CI: 23.2, 27.9) vs IR 18.1 (95% CI: 17.7, 18.5), inefficacy: IR 8.4 (95% CI: 7.2, 9.9) vs IR 7.4 (95% CI: 7.2, 7.7) and adverse events: IR 11.8 (95% CI: 10.3, 13.6) vs IR 7.1 (95% CI: 6.9, 7.4) (Table 2).
Fig. 1.
Kaplan–Meier estimates of crude persistence with TNFi therapy by age group
Table 2.
Incidence rate and Cox proportional hazard estimates (95% CI) for anti-TNF therapy discontinuation
| Age |
|||
|---|---|---|---|
| <75 yrs (n = 14 932) | ≥75 yrs (n = 768) | Total | |
| Number of subjects | 14 932 | 768 | 15 700 |
| Patients with TNFi failure, n (% of cohort) | 7756 (51.9) | 450 (58.6) | 8206 (52.3) |
| Reason for TNFi failure, n (%) | |||
| Inefficacy | 3193 (41.2) | 149 (33.1) | 3342 (40.7) |
| Adverse effect | 3044 (39.3) | 209 (46.4) | 3253 (39.6) |
| Remission | 51 (0.07) | 5 (1.1) | 56 (0.7) |
| Other | 1171 (15.1) | 75 (16.7) | 1246 (15.2) |
| Missing | 297 (3.8) | 12 (2.7) | 309 (3.8) |
|
| |||
| TNFi failure – all cause | |||
| Follow up (person-years) | 42 876 | 1766 | 44 642 |
| No. of TNFi patients with TNFi failures | 7756 | 450 | 8206 |
| Incidence rate / 100 patient years (95% CI) | 18.1 (17.7, 18.5) | 25.5 (23.2, 27.9) | 18.4 (18.0, 18.8) |
| Hazard ratio (95% CI) (ref methotrexate) | |||
| Unadjusted; monotherapy | 1.11 (1.05, 1.17)* | 1.13 (0.90, 1.41) | 1.12 (1.06, 1.18)* |
| Adjusted (imputed); monotherapy | 1.07 (1.01, 1.13)∗∗ | 1.15 (0.91, 1.45) | 1.08 (1.02, 1.14)∗∗ |
| Propensity (imputed); monotherapy | 1.06 (1.00, 1.12) | 1.12 (0.90, 1.40) | 1.06 (1.01, 1.13)∗∗ |
|
| |||
| TNFi failure – inefficacy | |||
| Follow up (Person-years) | 42 876 | 1766 | 44 642 |
| No. of TNFi patients with TNFi inefficacy | 3193 | 149 | 3342 |
| Incidence rate / 100 patient years (95% CI) | 7.4 (7.2, 7.7) | 8.4 (7.2, 9.9) | 7.5 (7.2, 7.7) |
| Hazard ratio (95% CI) (ref methotrexate) | |||
| Unadjusted; monotherapy | 1.06 (0.97, 1.16) | 0.66 (0.43, 0.99)∗∗ | 1.03 (0.95, 1.13) |
| Adjusted (imputed); monotherapy | 1.06 (0.97, 1.16) | 0.63 (0.41, 0.97)∗∗ | 1.03 (0.94, 1.13) |
| Propensity (imputed); monotherapy | 1.06 (0.97, 1.16) | 0.69 (0.45, 1.04) | 1.04 (0.95, 1.13) |
|
| |||
| TNFi failure – adverse events | |||
| Follow up (person-years) | 42 876 | 1766 | 44 642 |
| No. of TNFi patients with TNFi inefficacy | 3044 | 209 | 3253 |
| Incidence rate / 100 patient years (95% CI) | 7.1 (6.9, 7.4) | 11.8 (10.3, 13.6) | 7.3 (7.0, 7.5) |
| Hazard ratio (95% CI) (ref MTX) | |||
| Unadjusted; monotherapy | 1.21 (1.11, 1.32)* | 1.41 (1.02, 1.96)∗∗ | 1.23 (1.13, 1.34)* |
| Adjusted (imputed); monotherapy | 1.13 (1.03, 1.23)* | 1.46 (1.05, 2.03)∗∗ | 1.14 (1.05, 1.25)* |
| Propensity (imputed); monotherapy | 1.11 (1.02, 1.22)* | 1.35 (0.97, 1.88) | 1.13 (1.04, 1.23)* |
Adjusted for age, gender, disease duration, Rheumatic Disease Comorbidity Index, smoking, DAS28, HAQ-DI and steroid use.
Reference group = TNFi MTX combination. Supplementary Table S5, available at Rheumatology online reports on the hazard estimates for TNFi discontinuation by choice of combination therapy including TNFi-sulfasalazine, TNFi-leflunomide or TNF-multiple csDMARDs.
P-value <0.01,
P-value <0.05.
TNFi: TNF inhibitor.
Overall, patients receiving TNFi monotherapy were more likely to discontinue TNF blockade compared with patients receiving TNFi MTX combination therapy [hazard rate (HR) 1.12 (95% CI: 1.06, 1.18) P < 0.001]. This finding was maintained when restricting the analysis to the younger cohort but not the older cohort, with no statistically significant difference in the hazard rate for discontinuation between TNFi monotherapy and TNFi MTX combination (Fig. 2).
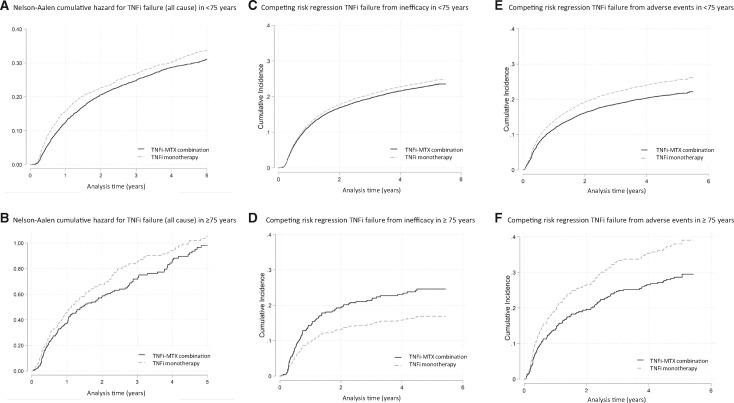
Fig. 2.
Cumulative hazard estimates of TNFi failure in patients on TNFi monotherapy and TNFi MTX combination therapy, by cause and by age
(A) Nelson-Aalen cumulative hazard for TNFi failure from all cause in patients <75 years old on TNFi monotherapy and TNFi MTX combination therapy. (B) Nelson-Aalen cumulative hazard for TNFi failure from all cause in patients ≥75 years old on TNFi monotherapy and TNFi MTX combination therapy. (C) Competing risk regression with cumulative incidence of TNFi failure from inefficacy in patients <75 years old on TNFi monotherapy and TNFi MTX combination therapy. (D) Competing risk regression with cumulative incidence of TNFi failure from inefficacy in patients ≥75 years old on TNFi monotherapy and TNFi MTX combination therapy. (E) Competing risk regression with cumulative incidence of TNFi failure from adverse events in patients <75 years old on TNFi monotherapy and TNFi MTX combination therapy. (F) Competing risk regression with cumulative incidence of TNFi failure from adverse events in patients ≥75 years old on TNFi monotherapy and TNFi MTX combination therapy.
When examining TNFi discontinuation by cause, patients in the ≥75 cohort receiving TNFi monotherapy were 34% less likely to discontinue TNFi due to inefficacy compared with patients receiving TNFi MTX combination [HR 0.66 (0.43–0.99) P = 0.04]. This finding was not seen in the younger cohort. Patients <75 years old receiving TNFi monotherapy were 6% more likely to discontinue TNFi due to inefficacy compared with patients receiving TNFi MTX, although this was not statistically significant. When examining TNFi discontinuation due to adverse events, patients in both age groups were more likely to discontinue therapy when prescribed TNFi monotherapy compared with TNFi MTX combination [≥75 HR 1.41 (1.02–1.96) P = 0.04] and [<75 HR 1.21 (1.11–1.32) P < 0.001] (Table 2 and Fig. 2).
There were no meaningful differences in point estimates from complete case analysis and those obtained using the imputed data (Supplementary Table S2, available at Rheumatology online). All results remained significant in the multivariable analyses. The PS model also had minimal influence on the point estimates, but the confidence included 1, indicating there may not sufficient evidence to conclude the observed difference is reliable in the over-75s (Supplementary Table S3, available at Rheumatology online).
Analyses investigating other TNFi/csDMARD combinations identified a greater risk of discontinuing TNF blockade in the <75 cohort if co-prescribed leflunomide compared with MTX [all cause: adjHR 1.22 (1·08–1.38) P = 0.001, and adverse event: adjHR 1.36 (1.13–1.63) P = 0.001]. Patients in this younger cohort were also less likely to discontinue anti-TNF if co-prescribed two csDMARDs compared with MTX alone [all cause: adjHR 0.86 (0.79–0.94) P <0.001, and adverse event: adjHR 0.85 (0.74–0.98) P = 0.02] (Supplementary Table S5, available at Rheumatology online).
Discussion
To our knowledge, this is the first study to investigate drug survival rates with TNFi monotherapy compared with TNFi/csDMARD combination therapy in older adults. In this large observational cohort of 15 000 patients, TNFi monotherapy is associated with an increase in treatment failure. However, in older adults (≥75 years) the disadvantage of TNFi monotherapy on drug survival is no longer seen. This is explained by fewer discontinuations due to inefficacy, but a greater risk of discontinuations due to adverse events. This could be interpreted as evidence that monotherapy is more acceptable in the elderly. An alternative narrative would be that we are observing a phenomenon of ‘competing risks’, an elderly patient may suffer an adverse event leading to termination of therapy, which removes the patient from the ‘risk pool’ prior to the outcome of interest, in this case, loss of drug efficacy.
We also demonstrated significant differences between csDMARD combination strategies. The use of two csDMARDs with TNF blockade is associated with improved drug survival in the younger cohort. However, the cohort was overwhelmingly made up of patients receiving MTX and/or sulfasalazine. Leflunomide was less frequently used, but its presence either alone or in combination had a negative association with TNF inhibitor drug survival, irrespective of age groups.
There are several possible explanations for our findings. Crucially, the adverse event signal seen with TNFi monotherapy compared with TNFi MTX combination therapy may be driven by channelling bias. Channelling is a form of selection bias seen in observational studies, where drugs with similar therapeutic indications are prescribed to groups of patients with prognostic differences [33]. It is plausible that patients with a greater risk of adverse events are more likely to be prescribed TNFi monotherapy which is presumed to have a better safety profile than combination therapy. To address for channelling bias in this cohort, a PS model was created. The technique allows the comparison of non-randomised treatment strategies, adjusting for known covariates that may predict treatment decisions. Despite this, unmeasured confounding likely remains.
In the ≥75-year-old cohort, the lower incidence of failure due to inefficacy with TNFi monotherapy is interesting and potentially of clinical relevance. This may reflect our a priori hypothesis that there is a reduction in immunogenicity in this age group, as the aging immune system becomes less effective at mounting antibody responses, as phenomenon known as immunosenescence [34]. Immunogenicity is a recognised mechanism underlying therapeutic failure with TNFi agents over time. Anti-drug antibodies are produced by the immune system in response to proteinaceous drugs, particularly monoclonal antibodies [35,36]. Concomitant use of MTX reduces the clearance of TNFi by lowering the incidence of anti-drug antibodies, resulting in a higher systemic exposure and improved drug survival. In the older cohort, a reduction in immunogenicity may improve TNFi drug survival and preclude the need for concomitant MTX. In support of this immunosenescence hypothesis, the reduced risk of TNFi discontinuation due to inefficacy in patients receiving monotherapy was no longer apparent in the exploratory analyses using a younger age cut-off of 65 and 70.
It is important to note that in our multivariate adjusted analyses, the imputed model demonstrated a statistically significant difference between the TNFi monotherapy and TNFi MTX combination, suggesting that the observed difference is not solely attributable to the measured confounders. However, in the imputed model with PS adjustment, the estimate was non-significant for the over 75s, though the difference in the point estimate between the two models was negligible. A plausible explanation for this is that there is confounding by indication. It is important to acknowledge that our adjustment model includes age and we may be including a path variable if our immunosenescence hypothesis is correct. It remains clear that age or some mechanism related to age is likely to be important in explaining the difference in effect of TNFi monotherapy vs combination therapy.
The effect size (adjusted hazard ratio of 0.63) suggests patients ≥75 receiving TNFi monotherapy are nearly 40% less likely to discontinue TNFi due to inefficacy compared with patients receiving TNFi MTX combination. In part, this may be explained by the competing risk phenomenon; some patients who were destined to fail due to inefficacy experience an adverse event before meeting the inefficacy end point, thereby selecting themselves out of the ‘at risk of inefficacy’ cohort. Older patients are more likely to stop TNFi therapy than younger patients, and adverse events is the highest contributing reason for discontinuation. This may explain the slightly paradoxical finding that fewer older people stop due to inefficacy on monotherapy. The finding of higher discontinuation rates in the elderly is not surprising. Age is a consistent predictor for many outcomes that may lead to discontinuation, such as infection or cancer and direct drug toxicity.
Our results are in keeping with published data from observational studies. The Dutch and Swiss registries reported comparable drug survival and reasons for discontinuations between the young and the elderly [14, 16], while the Italian registry demonstrated greater discontinuation in the elderly, with more frequent adverse events [17]. Zhang et al. demonstrated that concomitant MTX improves persistence to biologic therapy in patients over 65 years, although analyses included patients <65 years old with certain disabilities, and no information was provided regarding reasons for discontinuation [37]. In contrast to earlier analyses using BSRBR data, we did not demonstrate inferiority of the sulfasalazine/TNFi combination [9]. We did however confirm the association with leflunomide and lower TNFi treatment survival, which has also been demonstrated in the German registry, although this did not reach statistical significance [38].
This study has several strengths. The large sample size, limited missing data and accurate coding of treatment discontinuation has facilitated an in-depth and robust analysis. The BSRBR-RA includes data on elderly patients who are frequently excluded from clinical trials and provides real world data improving generalisability to clinical practice.
Despite the large overall sample size, the size of the ≥75-year cohort was relatively small, particularly in the ‘inefficacy’ model that limits statistical power. The decision to stop anti-TNF therapy and the reason for discontinuation was provided by the supervising rheumatologist, and we are unable to externally verify the accuracy of data provided. This may account for the number of ‘other’ or ‘missing’ entrees, possibly introducing a degree of misclassification bias. All our analyses were based on csDMARD regimen at study entry. Patients may modify their csDMARD regimen after the introduction of TNFi. During the 5-year observation period, 18% of the cohort changed from their initial therapy choice of TNFi monotherapy, TNFi MTX combination or TNFi-other csDMARD combination. Six percent of the cohort switched between TNFi monotherapy and TNFi MTX combination. The proportion of ‘switchers’ was similar between the two age cohorts. We did not consider patients who switched between initial therapy choice in our analyses and this may have influenced TNFi survival. We chose to exclude previous csDMARD exposure from our multivariate model despite recognising this as an important confounder. This is because prior csDMARD therapy associates with our predictor variable (i.e. being on TNFi monotherapy is more likely to be associated with multiple failed csDMARDs). Lastly, in this analysis we tested multiple hypotheses, which potentially increases the chances of a false-positive association, and as such our results should be interpreted with caution. Replicating these analyses in other registries’ data and corroborating our results would prove invaluable.
In conclusion, these data provide evidence to support TNFi monotherapy strategies in the over-75s in the wider context of a desire to reduce polypharmacy burden, the findings in this study should help alleviate physician concerns about drug immunogenicity in older patients.
Supplementary Material
Acknowledgements
The BSRBR-RA is a UK-wide national project to investigate the safety of biologic agents in routine medical practice. This work was supported by the British Society for Rheumatology (BSR), which receives restricted income from UK pharmaceutical companies, presently AbbVie, Celltrion, Hospira, Pfizer, Union Chimique Belge and Roche, and in the past Swedish Orphan Biovitrum and Merck. Disclaimers: The views expressed in the submitted article are the authors own and not an official position of their host institutions. The authors acknowledge support from the BSR Executive, the members of the BSR Registers and Research Committee and the BSRBR-RA Project Team for their active role in enabling the register to undertake its tasks. K.B. is funded by Medical Research Council as a Clinical Training Research Fellowship (CTRF- MR/R001332/1 to K Bechman)
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: J.B.G. has received honoraria for speaking or attending conferences from Pfizer, Bristol-Myers Squibb, UCB and Celgene. A.P.C. is a Trustee of the Kennedy Trust for Rheumatology Research, which has received royalty income linked to anti-TNF therapy when used in combination with methotrexate. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Pincus T, Yazici Y, Sokka T, Aletaha D, Smolen JS.. Methotrexate as the “anchor drug” for the treatment of early rheumatoid arthritis. Clin Exp Rheumatol 2003;21(5 Suppl 31):S179–85. [PubMed] [Google Scholar]
- 2. Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB. et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. [DOI] [PubMed] [Google Scholar]
- 3. Klareskog L, van der Heijde D, de Jager JP. et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675–81. [DOI] [PubMed] [Google Scholar]
- 4. Emery P, Fleischmann RM, Moreland LW. et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum 2009;60:2272–83. [DOI] [PubMed] [Google Scholar]
- 5. Keystone EC, Genovese MC, Klareskog L. et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis 2009;68:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emery P, Burmester GR, Bykerk VP. et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis 2015;74:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gomez-Reino J. Biologic monotherapy as initial treatment in patients with early rheumatoid arthritis. Rheumatology 2012;51(Suppl 5):v31–7. [DOI] [PubMed] [Google Scholar]
- 8. Kalden JR, Schulze-Koops H.. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol 2017;13:707–18. [DOI] [PubMed] [Google Scholar]
- 9. Soliman MM, Ashcroft DM, Watson KD. et al. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2011;70:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zink A, Listing J, Kary S. et al. Treatment continuation in patients receiving biological agents or conventional DMARD therapy. Ann Rheum Dis 2005;64:1274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jørgensen TS, Kristensen LE, Christensen R. et al. Effectiveness and drug adherence of biologic monotherapy in routine care of patients with rheumatoid arthritis: a cohort study of patients registered in the Danish biologics registry. Rheumatology 2015;54:2156–65. [DOI] [PubMed] [Google Scholar]
- 12. Souto A, Maneiro JR, Gómez-Reino JJ.. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology 2016;55:523–34. [DOI] [PubMed] [Google Scholar]
- 13. Kristensen LE, Saxne T, Nilsson JA, Geborek P.. Impact of concomitant DMARD therapy on adherence to treatment with etanercept and infliximab in rheumatoid arthritis. Results. From a six-year observational study in southern Sweden. Arthritis Res Ther 2006;8:R174.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Radovits BJ, Kievit W, Fransen J. et al. Influence of age on the outcome of antitumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis 2009;68:1470–3. [DOI] [PubMed] [Google Scholar]
- 15. Hyrich KL, Watson KD, Silman AJ, Symmons DP; The BSR Biologics Register. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology 2006;45:1558–65. [DOI] [PubMed] [Google Scholar]
- 16. Genevay S, Finckh A, Ciurea A. et al. Tolerance and effectiveness of anti-tumor necrosis factor alpha therapies in elderly patients with rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2007;57:679–85. [DOI] [PubMed] [Google Scholar]
- 17. Filippini M, Bazzani C, Favalli EG. et al. Efficacy and safety of anti-tumour necrosis factor in elderly patients with rheumatoid arthritis: an observational study. Clin Rev Allergy Immunol 2010;38:90–6. [DOI] [PubMed] [Google Scholar]
- 18. Hetland ML, Christensen IJ, Tarp U. et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum 2010;62:22–32. [DOI] [PubMed] [Google Scholar]
- 19. Krams T, Ruyssen-Witrand A, Nigon D. et al. Effect of age at rheumatoid arthritis onset on clinical, radiographic, and functional outcomes: the ESPOIR cohort. Joint Bone Spine 2016;83:511–5. [DOI] [PubMed] [Google Scholar]
- 20. Busquets N, Tomero E, Descalzo MÁ. et al. Age at treatment predicts reason for discontinuation of TNF antagonists: data from the BIOBADASER 2.0 registry. Rheumatology 2011;50:1999–2004. [DOI] [PubMed] [Google Scholar]
- 21. Agarwal S, Busse PJ.. Innate and adaptive immunosenescence. Ann Allergy Asthma Immunol 2010;104:183–90. [DOI] [PubMed] [Google Scholar]
- 22. Rink L, Cakman I, Kirchner H.. Altered cytokine production in the elderly. Mech Ageing Dev 1998;102:199–209. [DOI] [PubMed] [Google Scholar]
- 23. Panda A, Arjona A, Sapey E. et al. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol 2009;30:325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Siegrist CA, Aspinall R.. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 2009;9:185–94. [DOI] [PubMed] [Google Scholar]
- 25. Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB.. Age effects on B cells and humoral immunity in humans. Ageing Res Rev 2011;10:330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boraschi D, Aguado MT, Dutel C. et al. The gracefully aging immune system. Sci Transl Med 2013;5:185ps8.. [DOI] [PubMed] [Google Scholar]
- 27. Pawelec G. Immunosenescence comes of age. Symposium on aging research in immunology: the impact of genomics. EMBO Rep 2007;8:220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watson K, Symmons D, Griffiths I, Silman A.. The British Society for Rheumatology Biologics Register. Ann Rheum Dis 2005;64(Suppl 4):iv42–iv3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Michaud K, Wolfe F.. Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheumatol 2007;21:885–906. [DOI] [PubMed] [Google Scholar]
- 30. Wolfe F, Michaud K, Li T, Katz RS.. Chronic conditions and health problems in rheumatic diseases: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, systemic lupus erythematosus, and fibromyalgia. J Rheumatol 2010;37:305–15. [DOI] [PubMed] [Google Scholar]
- 31. England BR, Sayles H, Mikuls TR, Johnson DS, Michaud K.. Validation of the rheumatic disease comorbidity index. Arthritis Care Res 2015;67:865–72. [DOI] [PubMed] [Google Scholar]
- 32. Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 33. Petri H, Urquhart J.. Channeling bias in the interpretation of drug effects. Stat Med 1991;10:577–81. [DOI] [PubMed] [Google Scholar]
- 34. Jani M, Barton A, Warren RB, Griffiths CEM, Chinoy H.. The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology 2014;53:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bartelds GM, Krieckaert CL, Nurmohamed MT. et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 2011;305:1460–8. [DOI] [PubMed] [Google Scholar]
- 36. Pascual-Salcedo D, Plasencia C, Ramiro S. et al. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology 2011;50:1445–52. [DOI] [PubMed] [Google Scholar]
- 37. Zhang J, Xie F, Delzell E. et al. Impact of biologic agents with and without concomitant methotrexate and at reduced doses in older rheumatoid arthritis patients. Arthritis Care Res 2015;67:624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Strangfeld A, Hierse F, Kekow J. et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis 2009;68:1856–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.