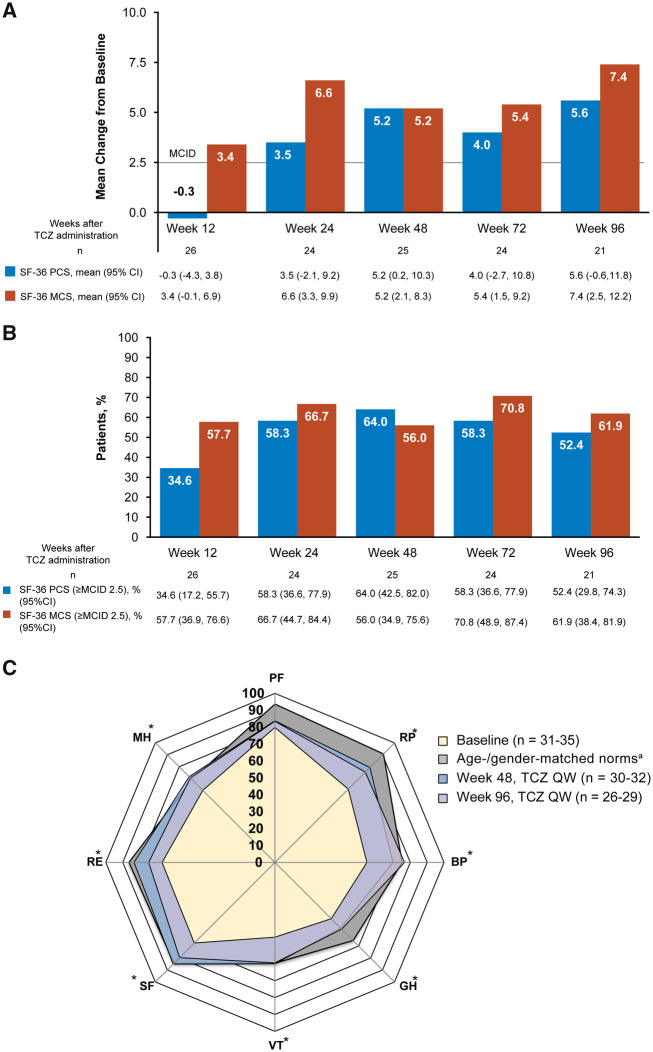
Fig. 2.
Patient well-being in the TAKT study
(A) Patient-reported outcomes measured by the SF-36 component summary scores. (B) Patients with clinically meaningful improvements (MCID >2.5) from baseline. (C) Individual domain scores. Patients with missing baseline data were excluded for these analyses. *Change from baseline MCID of >5.0 for TCZ QW at weeks 48 and 96. aFor calculation of age- and gender-matched norms, patients younger than 20 years of age were calculated as 20 years of age. MCS consists of social functioning, mental health, role emotional and vitality domains. PCS consists of physical functioning, role physical, bodily pain and general health domains. SF-36 MCID has not been validated in patients with TAK. BL: baseline; BP: bodily pain; GH: general health; MCID: minimum clinically important difference; MCS: mental component summary; MH: mental health; PCS: physical component summary; PF: physical function; QW: once weekly; RE: role emotional; RP: role physical; SF: social function; SF-36: 36-Item Short Form Health Survey; TAKT: Takayasu arteritis Treated with Tocilizumab trial; TCZ: tocilizumab; VT: vitality.