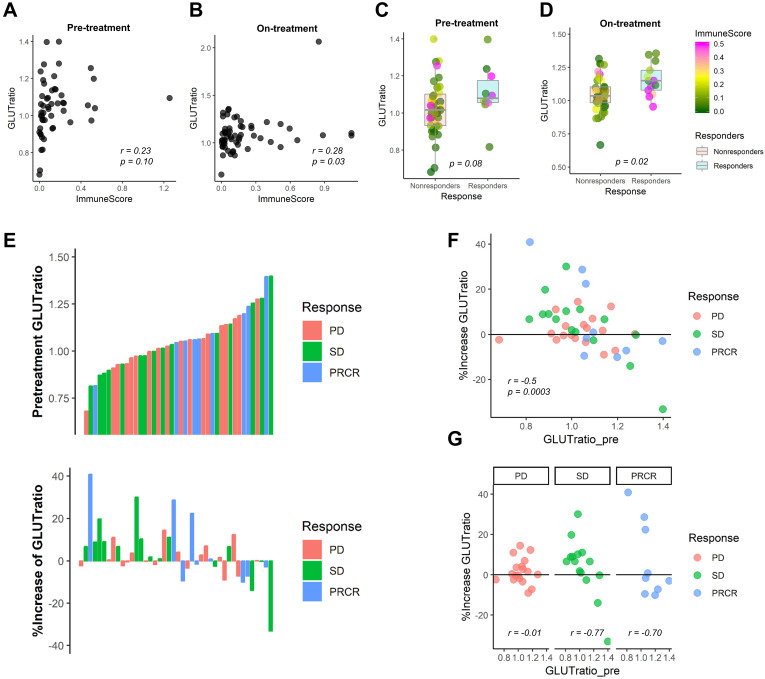
Figure 5.
The ratio of GLUT3 to GLUT1 as a surrogate marker for evaluating metabolic functionality of immune cells in melanoma patients treated with immunotherapy. From the RNA-sequencing data from melanoma patients who underwent anti-PD-1 treatment, we examined the GLUT-ratio, the ratio of GLUT3 to GLUT1, whether it could predict treatment response. (A, B) The GLUT-ratio showed a positive correlation with ImmuneScore in pre-treatment (r = 0.23, p = 0.10) as well as on-treatment (r = 0.28, p = 0.03) melanoma tissues. (C, D) The GLUT-ratio of nonresponders (stable disease and progressive disease) and responders (complete or partial remission) was compared. (C) There was a trend of higher GLUT-ratio in responders compared with nonresponders in pre-treatment phase (p = 0.08). (D) This trend was more prominent in the on-treatment phase. The GLUT-ratio of responders and nonresponders was significantly different in on-treatment phase (p = 0.02). (E) The relationship between anti-PD-1 treatment response according to RECIST criteria and pre-treatment GLUT-ratio, %change of GLUT-ratio after the immunotherapy treatment was presented. (F) A scatter plot depicts a relationship between GLUT-ratio from pre-treatment tissue and the % change of GLUT-ratio after the anti-PD-1 treatment. Overall, there was a significant negative correlation between %change and baseline value of the GLUT-ratio (r = -0.50, p = 0.0003), which suggests GLUT-ratio is increased after the treatment in tumors with low pretreatment GLUT-ratio. (G) According to each response criteria, the patients with SD or PR/CR showed a negative correlation, however, the patients with PD did not show any correlation between two parameters, pretreatment and %change of GLUT-ratio (r = -0.77 for SD; r = -0.70 for PR/CR; r = -0.01 for PD). Specifically, there was very few patients who showed GLUT-ratio increment among low GLUT-ratio in patients with PD. (irRECIST = immune-related response evaluation criteria in solid tumors; CR = complete remission; PR = partial remission; SD = stable disease; PD = progressive disease).