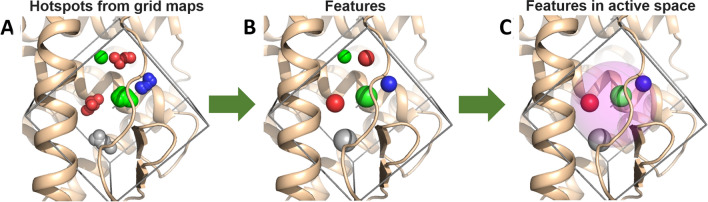
Fig. 2.
Pharmacophore building. Pharmacophores are obtained from the binding-site hotspots. a Hotspots are identified by clustering the grids with highest x% percentage of affinity-energy from the affinity maps (enclosed in the box). e.g., for 5 atom types (H-bond acceptor (red), H-bond donors (blue), hydrophobic (green), aromatic (gray) and charge atoms (not shown for sake of simplicity)) are shown. b Then, the center of mass (energy-weighted) and the radius of gyration, for each hotspot, are calculated, and used to define the pharmacophoric features (atom type, center of mass and the radius of gyration). c Finally, the set of pharmacophores is obtained from all possible combinations of 3 features which centers of mass are located inside of a predefined sphere (pink) of radius 5 Å (active space) that is centered at the grid-map center. In this study, three active spaces were used in each binding site. Therefore, the process (involving the steps A, B and C) was repeated for three sets of grid affinity maps with different centers