Fig. 1. Demonstration of catalytic degradation of targeted proteins by reversible covalent PROTACs.
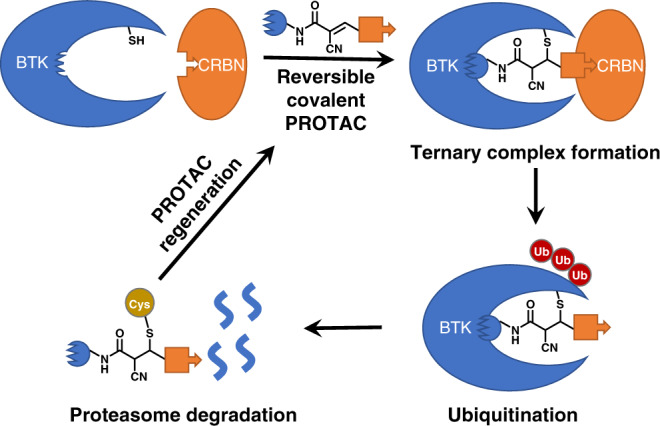
The premise of this reversible covalent PROTAC design is the weak reactivity (mM Kd) between α-cyano-acrylamide group (the chemical structure shown above) and free thiols. Only when the PROTAC molecule binds to the active site of the targeted protein, the nearby cysteine side chain can react with the α-cyano-acrylamide group to form a stable covalent bond. Once the targeted protein is degraded, the reversible covalent PROTAC can be regenerated.
