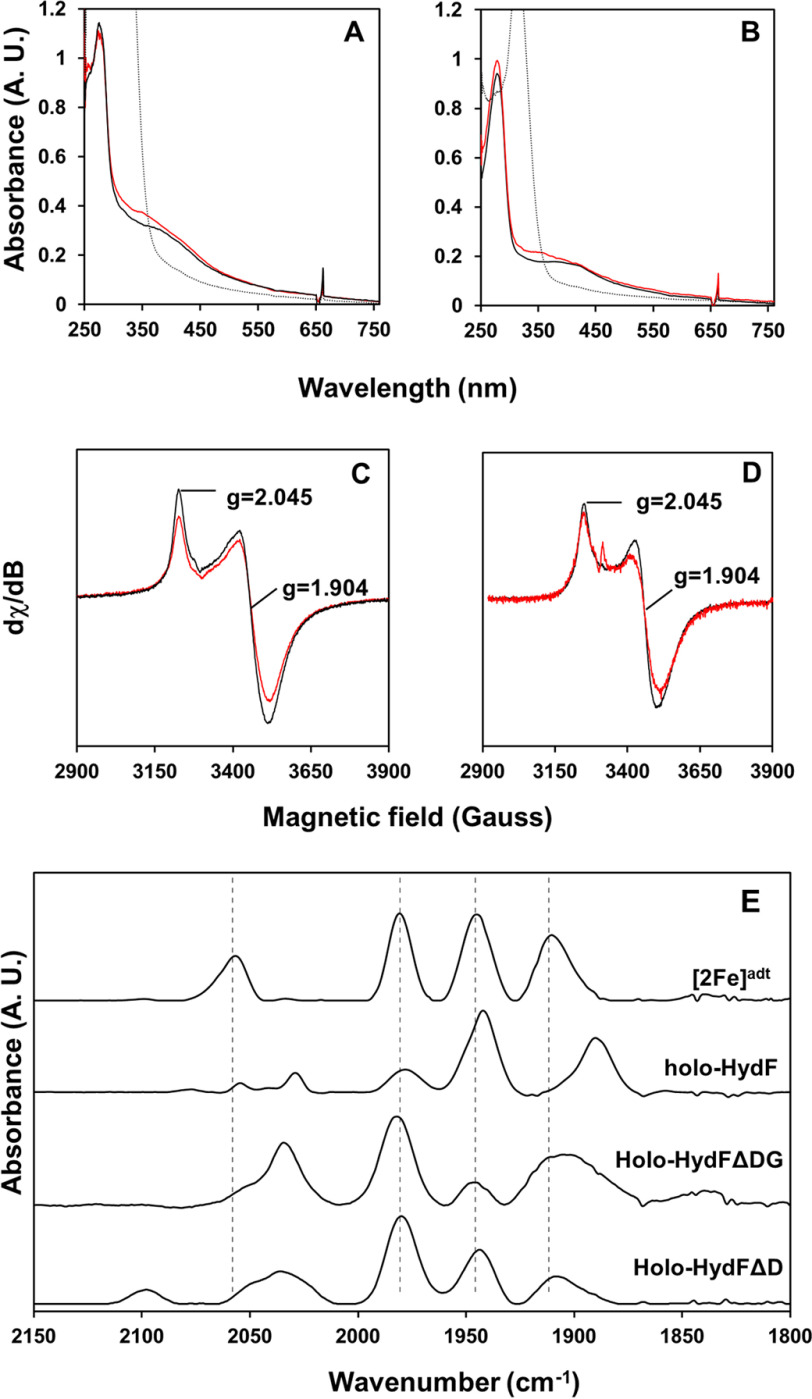
Figure 6.
Spectroscopic characterization of the truncated HydF proteins. A and B, UV-visible spectra of HydFΔD (A) and HydFΔDG (B). Shown are the reconstituted ([4Fe-4S]2+) form of the HydF variants (black spectra), the [2Fe]adt cofactor-loaded holo-forms ([2Fe]adt-[4Fe-4S]2+) (red spectra), and the Na-DT reduced ([4Fe-4S]+) forms (dashed spectra). The samples were prepared in a buffer containing 100 mm Tris-HCl and 300 mm KCl with a protein concentration of 100 μm (HydFΔD) or 50 μm (HydFΔDG). Na-DT (0.5 mm) was added to generate the reduced samples. C and D, low-temperature EPR spectra of the reduced forms of HydFΔD (200 μm) (C) and HydFΔDG (200 μm) (D). Shown are both reconstituted (black spectra) and [2Fe]adt-loaded forms (red spectra), and observed g-values are indicated. E, FTIR spectra of the [2Fe]adt-loaded proteins, holo-HydFΔD and HydFΔDG. Spectra recorded for [2Fe]adt and holo-HydF are displayed for comparison, and the peak positions of [2Fe]adt are indicated with vertical dashed lines. The EPR spectra were recorded at 10 K, 1 mW microwave power, 10-Gauss modulation amplitude, and 100-kHz modulation frequency. The microwave frequency was 9.28 GHz. The FTIR spectra were recorded at room temperature, and the samples were prepared in a solution containing approximately 2.5 mm protein, 100 mm Tris-HCl, pH 8.0, 300 mm KCl.