Figure 6.
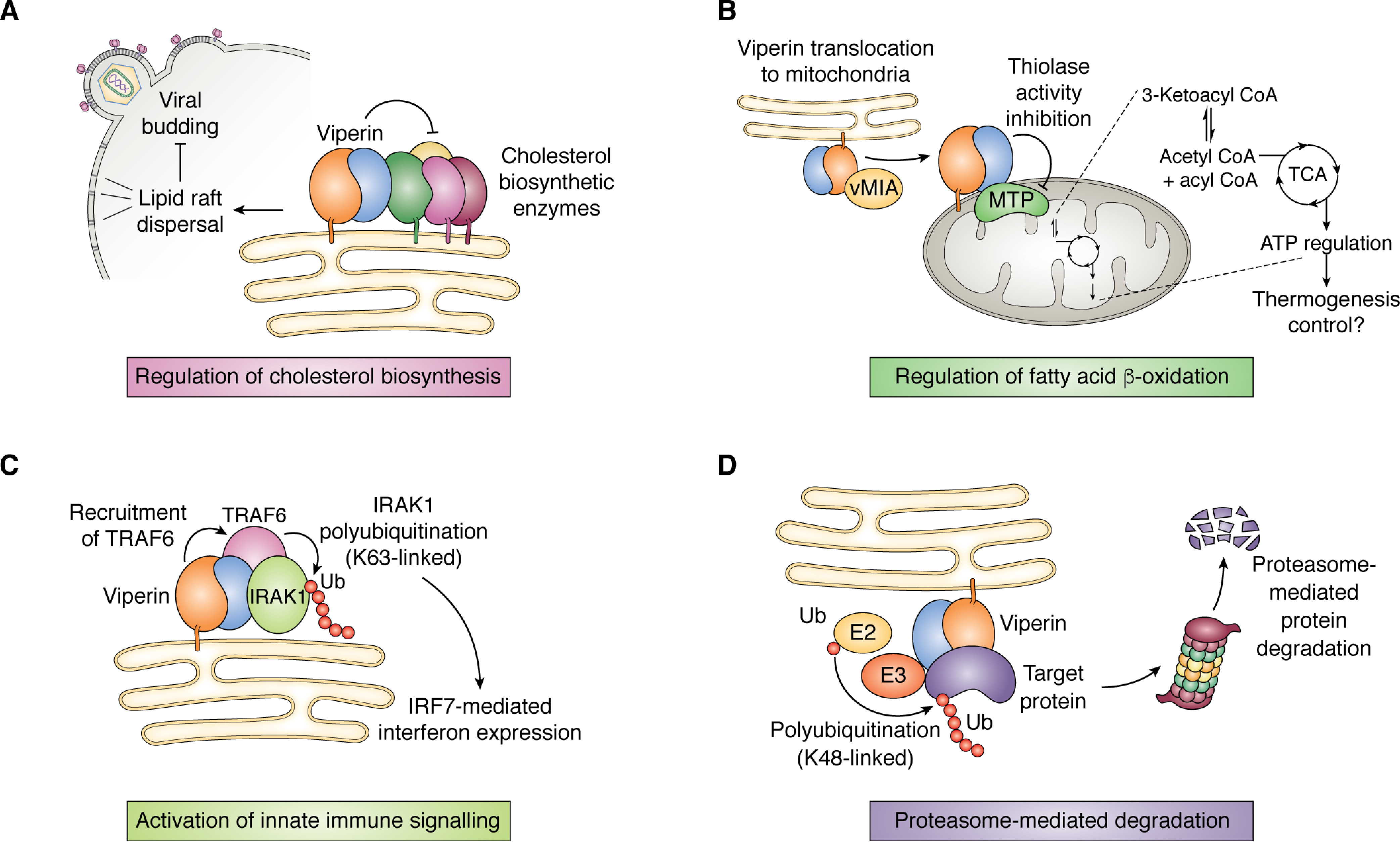
Overview of viperin's interaction with other proteins. A, viperin perturbs the formation of cholesterol-rich lipid rafts, which stalls budding of enveloped viruses such as influenza A. This is thought to occur through the down-regulation of cholesterol biosynthetic enzymes, including FPPS. B, translocation of viperin to the mitochondrion results in down-regulation of the thiolase activity of HADHB, the β-subunit of the MTP complex involved in fatty acid β-oxidation, which impacts ATP production. C, viperin interacts with the protein kinase IRAK1 and E3 ubiquitin ligase TRAF6 to facilitate Lys-63–linked polyubiquitination of IRAK1 by TRAF6, thereby activating the IRF7-mediated interferon expression. D, viperin interacts with various structural and nonstructural viral proteins and promotes their degradation through the ubiquitin-dependent proteasomal pathway.
