Figure 2.
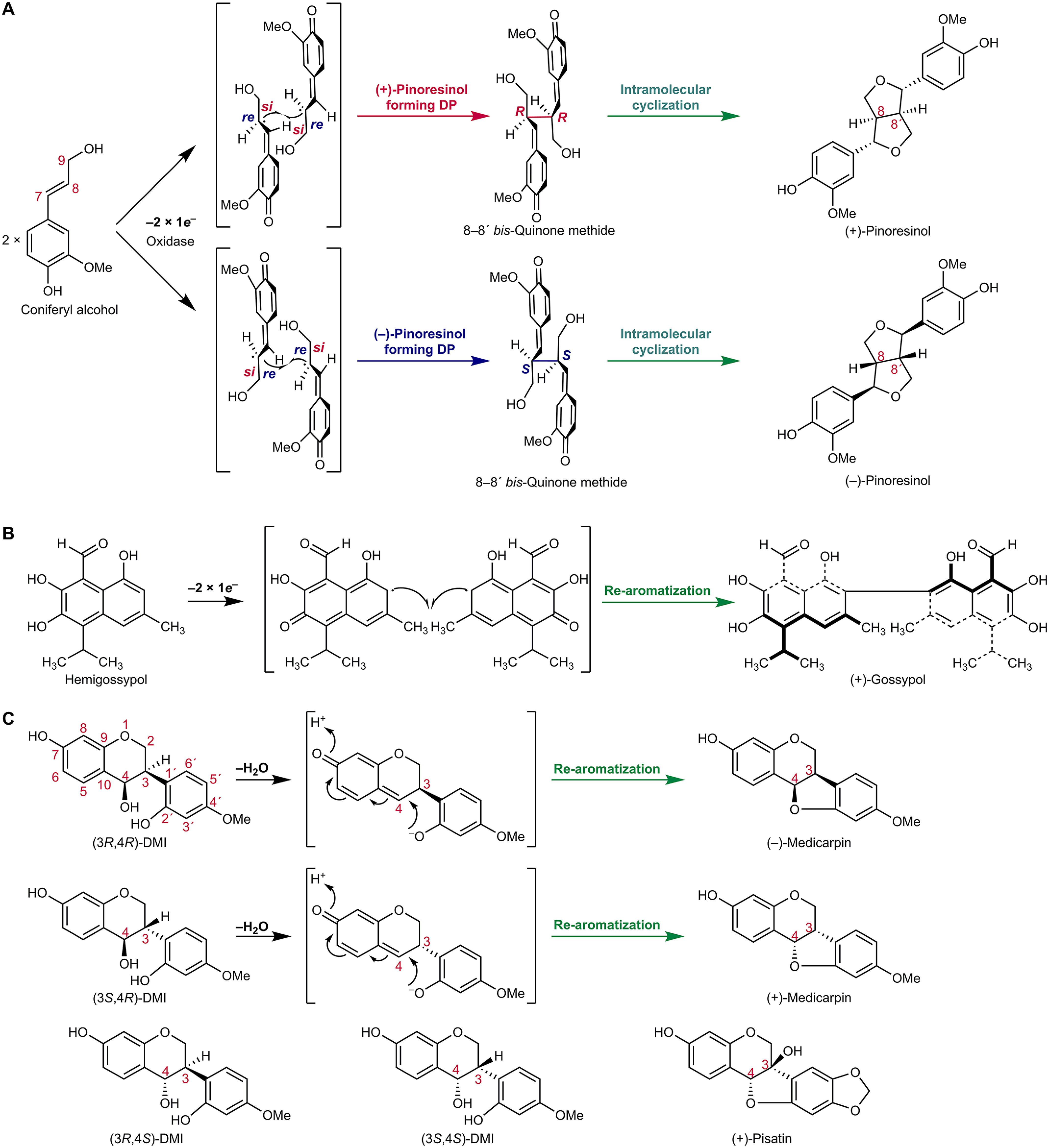
Proposed general biochemical mechanism of DPs involving generation and stabilization of mono- or bis-quinone methides. A, formation of either (+)- or (–)-pinoresinol from achiral coniferyl alcohol. Initially, 1e– oxidation generates an intermediary prochiral free radical mono-quinone methide, which undergoes either si-si or re-re coupling to afford the chiral 8–8′-bis-quinone methides, depending on the pinoresinol-forming DP, to give after intramolecular cyclization either (+)- or (–)-pinoresinol, respectively. B, (+)-gossypol–forming DP whose action requires 1e– oxidation of achiral hemigossypol to afford the proposed prochiral free radical mono-quinone methide intermediate. Stereoselective coupling then gives the bis-quinone methide derivative, re-aromatization of which generates (+)-gossypol. C, medicarpin-forming DP using chiral isoflavonoid substrates (3R,4R)-DMI, and (3S,4R)-DMI. The proposed biochemical mechanism involves mono-quinone methide generation and intramolecular cyclization/re-aromatization. (3R,4S)-DMI and (3S,4S)-DMI are poorer substrates. (+)-Pisatin is another example of a pterocarpan.
