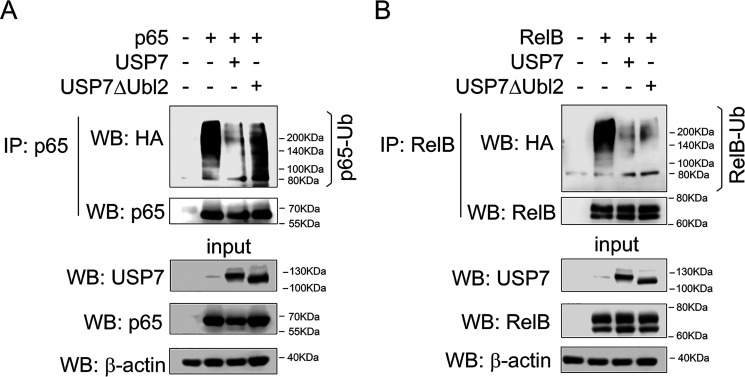
Figure 4.
The Ubl2 domain is required for USP7 deubiquitination of p65 but not RelB. HEK293T cells were transfected with HA-tagged ubiquitin, USP7, USP7ΔUbl2, and p65 (A) or RelB (B). Denatured whole cell lysates were immunoprecipitated with anti-p65 (A) or anti-RelB (B) and analyzed by Western blotting (WB) with anti-HA antibody and anti-p65 (A) or anti-RelB (B) antibody. The expression of transfected USP7, USP7ΔUbl2, p65, and RelB in lysates used for immunoprecipitations (IP, input) was measured by Western blotting using antibodies against USP7, p65, and RelB. The positions of molecular mass markers are indicated to the right of each Western blot. The data are representative of at least three independent experiments.