Abstract
BACKGROUND
Currently used pre-operative prediction scores for postoperative pulmonary complications (PPCs) use patient data and expected surgery characteristics exclusively. However, intra-operative events are also associated with the development of PPCs.
OBJECTIVE
We aimed to develop a new prediction score for PPCs that uses both pre-operative and intra-operative data.
DESIGN
This is a secondary analysis of the LAS VEGAS study, a large international, multicentre, prospective study.
SETTINGS
A total of 146 hospitals across 29 countries.
PATIENTS
Adult patients requiring intra-operative ventilation during general anaesthesia for surgery.
INTERVENTIONS
The cohort was randomly divided into a development subsample to construct a predictive model, and a subsample for validation.
MAIN OUTCOME MEASURES
Prediction performance of developed models for PPCs.
RESULTS
Of the 6063 patients analysed, 10.9% developed at least one PPC. Regression modelling identified 13 independent risk factors for PPCs: six patient characteristics [higher age, higher American Society of Anesthesiology (ASA) physical score, pre-operative anaemia, pre-operative lower SpO2 and a history of active cancer or obstructive sleep apnoea], two procedure-related features (urgent or emergency surgery and surgery lasting ≥ 1 h), and five intraoperative events [use of an airway other than a supraglottic device, the use of intravenous anaesthetic agents along with volatile agents (balanced anaesthesia), intra-operative desaturation, higher levels of positive end-expiratory pressures > 3cmH2O and use of vasopressors]. The area under the receiver operating characteristic curve of the LAS VEGAS risk score for prediction of PPCs was 0.78 [95% confidence interval (95% CI), 0.76 to 0.80] for the development subsample and 0.72 (95% CI, 0.69 to 0.76) for the validation subsample.
CONCLUSION
The LAS VEGAS risk score including 13 peri-operative characteristics has a moderate discriminative ability for prediction of PPCs. External validation is needed before use in clinical practice.
TRIAL REGISTRATION
The study was registered at Clinicaltrials.gov, number NCT01601223.
Introduction
An estimated 230 million major surgical procedures are undertaken worldwide each year.1 Complications after major surgery occur frequently and are an important cause of mortality and morbidity,2,3 especially when they affect the lungs.4 Indeed, one in every seven patients who develops a so-called postoperative pulmonary complication (PPC) dies before hospital discharge, and patients who survive often suffer from a sustained reduction in functional status.1–3 Early identification of patients at risk of developing PPCs could enable the use of preventive measures as well as timely treatment.
The ‘Assess Respiratory Risk in Surgical Patients in Catalonia’ (ARISCAT) risk score2 and the ‘Surgical Lung Injury Prediction’ (SLIP) model5,6 are two prediction scores used for the identification of patients at risk of developing PPCs or the acute respiratory distress syndrome (ARDS), respectively. Both scores are composed of pre-operative patient characteristics, such as age and the presence of comorbidities, and pre-operative procedure-related features, such as type of surgery and expected duration of the surgical intervention,2,3,5 but fail to use intra-operative events, such as those related to intra-operative ventilation,7 and systemic circulation.8,9 Intra-operative events have also been found to have an association with postoperative outcomes, and incorporation of these in prediction models could thus strengthen predictability.10
We sought to develop and validate an improved prediction score, partly based on the above-mentioned ARISCAT prediction score and SLIP model, but using both pre-operative and intra-operative data. For this, we reanalysed the database of the ‘Local Assessment of Ventilatory Management During General Anesthesia for Surgery’ (LAS VEGAS) study. We hypothesised that the addition of intra-operative data would enhance predictability compared with existing models.
Materials and methods
This manuscript was reported according to the TRIPOD checklist.11
Source of data
This is a secondary analysis of the LAS VEGAS study, an international, multicentre, prospective, cross-sectional study that took place in 146 centres worldwide.12,13 The complete list of planned secondary analyses of LAS VEGAS is available in the Appendix and on the PROVENet website (www.provenet.eu). LAS VEGAS was registered at Clinicaltrials.gov (NCT01601223) and was endorsed, and partly funded by the European Society of Anaesthesiology (ESA). The Clinical Trial Network of the ESA assisted in developing the electronic case record forms and hosted the electronic database, but had no influence on study design, study conduct, data analysis and interpretation, nor final reporting.
Ethics
The study protocol was first approved by the ethics committee of the Academic Medical Center, Amsterdam, the Netherlands (W12_190#l 2.17.0227, approved on 22 August 2012; Chair mw. Dr M.D. Trip) and subsequently in each centre, as requested by national guidelines. Surgical patients were enrolled over a period of 7 consecutive days between 14 January and 4 March 2013.
Participants
Patients who fulfilled the following inclusion criteria were included in the LAS VEGAS study: age more than 18 years, and receiving invasive ventilation during general anaesthesia for elective or nonelective surgery. Patients were excluded if they were scheduled for pregnancy-related surgery, or underwent a surgical procedure outside the operating room. For this secondary analysis, we had the following additional exclusion criteria: surgery involving cardiopulmonary bypass and thoracic surgery or surgery involving one-lung ventilation. In addition, patients who had received ventilation at any time in the previous 30 days were also excluded. Finally, for the present analysis, we considered only patients for whom there were no missing values in the variables of interest.
Data collection
As described in detail elsewhere,12,13 baseline patient characteristics, including age, sex, weight, height, ASA physical score, functional status and comorbidities, were collected before surgery. During the intra-operative period, ventilator settings, including tidal volume, positive end-expiratory pressure (PEEP) and peak pressure, inspired fraction of oxygen (FiO2), respiratory rate and recruitment manoeuvres, and hourly-recorded vital signs, including heart rate and blood pressure, pulse oximetry readings and administration of unplanned vasoactive drugs were recorded.
PPCs were observed and recorded daily from the day of surgery (day 0) until discharge from hospital or until postoperative day five, whichever came first. Each adverse pulmonary event was recorded daily, but only counted once in the composite score. Length of hospital stay and in-hospital mortality was determined from patient records at postoperative day 28 as determined in the original protocol, and following common practice.12,13
Outcome
The primary endpoint was the development of PPCs during the first five postoperative days. This endpoint was a composite of unplanned supplementary oxygen, respiratory failure, unplanned new or prolonged invasive mechanical ventilation, ARDS, pneumonia and/or pneumothorax (eTable 1, http://links.lww.com/EJA/A162).
Definitions
The definitions for the following intra-operative events were desaturation, defined as SpO2 less than 92% for more than 2 min; hypotension, a SBP less than 90 mmHg for 3 min or longer; arrhythmia, new-onset atrial fibrillation, ventricular tachycardia, supraventricular tachycardia or ventricular fibrillation; and vasoactive support, infusion of any unplanned vasoactive drug.
Analysis plan
We divided the sample randomly into two cohorts using a computer algorithm without the influence of the researcher (using the function ‘sample’ from R, https://www.R-project.org). The development subsample (65% of patients) was used to construct a model, and the validation subsample (35%) was used to confirm its discriminatory capability.
Predictors
Potential predictors of PPCs were any of those used in previous studies on PPCs.2–6,14,15 The following predictors were considered for the initial multivariable model (after univariable selection as described below): sex, age, BMI, ASA physical score, smoker, functional status, pre-operative anaemia, respiratory infection, pre-operative SpO2, chronic obstructive pulmonary disease, cancer, chronic kidney disease, heart failure, obstructive sleep apnoea, condition of surgery, duration of surgery, use of supraglottic device, use of epidural anaesthesia, use of antibiotic prophylaxis, total fluid infusion, need for blood transfusion, type of anaesthesia, use of neuromuscular blocking agents, intraoperative desaturation, need for unplanned lung recruitment manoeuvre, intraoperative hypotension, arrhythmia, need of vasoactive drug, use of antagonists to neuromuscular blocking agents, level of PEEP, peak pressure and FiO2.
Sample size
The reported incidence of PPCs varies between 2.6 and 5.0%.2,14 We anticipated that to provide a sample of at least 120 PPC-events, the inclusion of at least 4800 patients in 96 centres would be required.12,13
Missing
As described above, we considered only patients for whom there were no missing values in the variables of interest.
Statistical analyses
Normally distributed data were described as mean ± standard deviation (SD); non-normally distributed data were reported as median and interquartile range (lower quartile to upper quartile). Categorical variables were reported as proportions (%). According to the distribution of the variables, the continuous variables were compared using independent or paired Student’s t tests; analysis of variance; Mann-Whitney test; or Kruskal-Wallis test. Categorical variables were compared using Chi-squared or Fisher exact tests.
The unadjusted association between the potential predictors and development of PPCs was assessed using multilevel univariable logistic regression models. Variables with P value less than 0.2 in this univariable model were selected for inclusion in the multivariable model. The multilevel multivariable logistic regression model was constructed using a backward stepwise selection procedure. Potential predictors were sequentially removed if this exclusion did not result in a significant change in the log-likelihood ratio test. The cutoff for variable removal used a significance level of 0.05. Linearity for each continuous variable was assessed and transformations applied where appropriate. In all multilevel models, the participating centres were treated as a random effect.16 We then calculated the adjusted odds ratios and the corresponding 95% confidence intervals (95% CIs) values. Calibration was formally assessed by the Hosmer-Lemeshow goodness of fit and by calibration plots.17
To avoid overfitting of the data for the development sample, a bootstrap method was used to find the best subset of factors. One thousand computer-generated samples were derived from the development subsample by random-selection with replacement.18 Within each bootstrap sample, the β coefficient was calculated using all selected independent variables. The reliability of predictor variables in the final regression model was estimated by the 80% CI of the β coefficient in the bootstrap samples. Reliable predictors were expected to be retained if the 80% CI of bootstrap samples indicated statistical significance (Ρ<0.05).
A predictive risk score was then calculated according to the following formula: P = ea+bX / 1 + ea+bX, where P is the predictive probability of development of PPCs, e is exponential, a is the intercept of the final model, b is the β coefficient of the logistic regression and X is the value of the variable.16 To assess the discriminative performance of this risk score in both the development and validation subsamples, we used the c-statistic, which was also displayed graphically as the area under the receiver operating characteristic (ROC) curve. An area under the ROC curve (AUC) of 0.5 indicates no discrimination, whereas an AUC of 1.0 indicates perfect discrimination. The area under the ROC of the LAS VEGAS score and the area under the ROC of the ARISCAT score were compared. A P value less than 0.05 means that the area under the ROC curves differ significantly.19
To increase the readiness of the score, we recalculated the final model with the continuous variables categorised according to their tertiles or based on previous cutoffs.1,5,14,15 Then, a simplified predictive risk score was calculated by multiplying each logistic β coefficient of regression by 10 and rounding off its value. The simplified score for development subsample cases were added together to produce an overall PPCs risk score for each patient. To evaluate the ability of the model to predict increasing rates of PPCs, we used that score and the minimum description length principle to divide the subsample into three ranges reflecting low, medium, and high risk for PPCs, each containing a similar number of patients with a PPC.
In another posthoc analysis, we tested the ability of the score in predicting severe PPCs (i.e. excluding ‘unplanned supplementary oxygen’). Finally, we also tested the predictive ability of the score after removing PEEP from it.
All analyses were conducted with R v.3.3.2 (http://www.R-project.org). For all analyses, two-sided P values less than 0.05 were considered significant.
Results
Participants
Of the 10520 patients enroled in 146 centres, 6063 patients were included in the present analyses (Fig. 1 and Table 1). Patients who developed one or more PPCs had a higher in-hospital mortality (3.2 vs. 0.3%; P < 0.001) and longer hospital length of stay (4 [1 to 5] vs. 2 [0 to 4]; P < 0.001). There was no difference between the cohort of patients who entered the final analysis and the cohort of patients excluded due to missing values of interest (eTable 2, http://links.lww.com/EJA/A162). The development and validation subsample were comparable with regard to case-mix and occurrence of PPCs (eTable 3 and eTable 4, http://links.lww.com/EJA/A162).
Fig. 1.
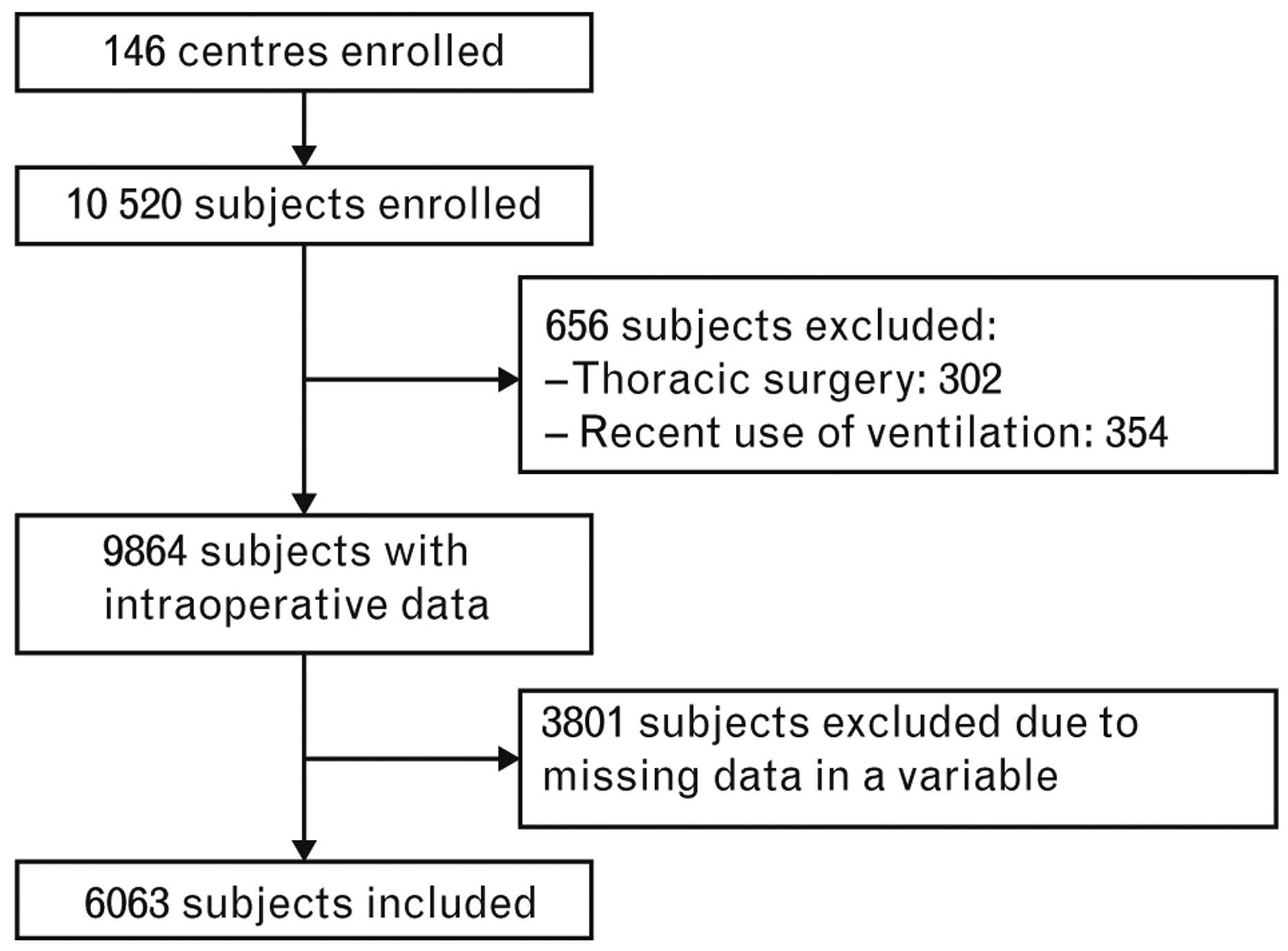
Flowchart of inclusion.
Table 1.
Characteristics of the included patients
| Development cohort (n = 3919) | Validation cohort (n = 2144) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 54 [41 to 67] | 55 [40 to 66] | 0.581 |
| Gender, male | 1778/3919 (45.4) | 931/2144 (43.4) | 0.145 |
| BMI (kg m−2) | 26.3 [23.3 to 29.9] | 26.2 [23.4 to 30.0] | 0.601 |
| ASA PS | 2 [1 to 2] | 2 [1 to 2] | 0.210 |
| 1 | 1058 / 3919 (27.0) | 613 / 2144 (28.6) | |
| 2 | 1954 / 3919 (49.9) | 1046 / 2144 (48.8) | |
| 3 | 826 / 3919 (21.1) | 459 / 2144 (21.4) | 0.087 |
| 4 | 80 / 3919 (2.0) | 24 / 2144 (1.1) | |
| 5 | 1 / 3919 (0.0) | 2 / 2144 (0.1) | |
| Functional status | |||
| Independent | 3629 / 3919 (92.6) | 1999 / 2144 (93.2) | |
| Partially dependent | 241 / 3919 (6.1) | 127 / 2144 (5.9) | 0.318 |
| Totally dependent | 49 / 3919 (1.3) | 18/2144 (0.8) | |
| ARISCAT score | 16 [3 to 26] | 15 [3 to 26] | 0.818 |
| < 26 | 2772 / 3919 (70.7) | 1530 / 2144 (71.4) | |
| 26–44 | 938 / 3919 (23.9) | 510 / 2144 (23.8) | 0.700 |
| > 44 | 209 / 3919 (5.3) | 104 / 2144 (4.9) | |
| Smoking | 930 / 3919 (23.7) | 495 / 2144 (23.1) | 0.572 |
| Preoperative SpO2, % | 98 [96 to 99] | 98 [96 to 99] | 0.448 |
| Preoperative anaemiaa | 129 / 3919 (3.3) | 79 / 2144 (3.7) | 0.421 |
| Respiratory infection < 30 days | 157 / 3919 (4.0) | 96 / 2144 (4.5) | 0.380 |
| Comorbidities | |||
| Cancer | 195 / 3919 (5.0) | 92 / 2144 (4.3) | 0.230 |
| Chronic kidney failure | 141 / 3919 (3.6) | 71 / 2144 (3.3) | 0.561 |
| COPD | 259 / 3919 (6.6) | 123 / 2144 (5.7) | 0.181 |
| Heart failure | 249 / 3919 (6.4) | 144 / 2144 (6.7) | 0.583 |
| Obstructive sleep apnoea | 76 / 3919 (1.9) | 41 / 2144 (1.9) | 0.941 |
| Neuromuscular diseaseb | 41 / 3919 (1.0) | 15/2144 (0.7) | 0.177 |
| Liver dysfunction | 46 / 3919 (1.2) | 22 / 2144 (1.0) | 0.601 |
| Surgical characteristics | |||
| Surgical procedureb | |||
| Lower gastrointestinal | 470 / 3919 (12.0) | 225 / 2144 (10.5) | 0.079 |
| Upper Gl, HB and pancreas | 567 / 3919 (14.5) | 337 / 2144 (15.7) | 0.191 |
| Vascular surgeryd | 121 / 3919 (3.1) | 98 / 2144 (4.6) | 0.003 |
| Aortic surgery | 27 / 3919 (0.7) | 24 / 2144 (1.1) | 0.079 |
| Neurosurgery and HN | 739 / 3919 (18.9) | 398 / 2144 (18.6) | 0.779 |
| Urological and kidney | 382 / 3919 (9.7) | 177 / 2144 (8.3) | 0.055 |
| Gynaecological | 435 /3919 (11.1) | 251 / 2144 (11.7) | 0.475 |
| Endocrine | 99 / 3919 (2.5) | 48 / 2144 (2.2) | 0.486 |
| Transplant | 19/3919 (0.5) | 9/2144 (0.4) | 0.721 |
| Plastic, cutaneous, breast | 436 /3919 (11.1) | 227 / 2144 (10.6) | 0.521 |
| Bone, joint, trauma, spine | 603 / 3919 (15.4) | 336 / 2144 (15.7) | 0.769 |
| Others | 204 / 3919 (5.2) | 140 / 2144 (6.5) | 0.033 |
| Surgical techniquec | |||
| Open | 765 / 3919 (19.5) | 426 / 2144 (19.9) | 0.743 |
| Laparoscopic | 742 / 3919 (18.9) | 376 / 2144 (17.5) | 0.180 |
| Laparoscopic assisted | 74 / 3919 (1.9) | 40 / 2144 (1.9) | 0.950 |
| Peripheral | 661 / 3919 (16.9) | 402 / 2144 (18.8) | 0.065 |
| Other | 1709 /3919 (43.6) | 915 / 2144 (42.7) | 0.484 |
| Condition of surgery | |||
| Elective | 3513 / 3919 (89.6) | 1919 / 2144 (89.5) | |
| Urgent | 319 / 3919 (8.1) | 176 / 2144 (8.2) | 0.981 |
| Emergency | 87 / 3919 (2.2) | 49 / 2144 (2.3) | |
| Duration of surgery (min) | 77 [47 to 130] | 75 [45 to 130] | 0.420 |
| Duration of anaesthesia (min) | 107 [74 to 169] | 105 [70 to 170] | 0.405 |
| Intraoperative characteristics | |||
| Use of supraglottic devices | 507 / 3919 (12.9) | 298 / 2144 (13.9) | 0.291 |
| Epidural anaesthesia | 230 / 3919 (5.9) | 122 / 2144 (5.7) | 0.776 |
| Fluid infused (ml) | 1000 [1000 to 1900] | 1000 [900 to 2000] | 0.139 |
| Blood transfusion | 161 / 3919 (4.1) | 79 / 2144 (3.7) | 0.418 |
| Use of opioid | 3902 / 3919 (99.6) | 2128 / 2144 (99.3) | 0.113 |
| Type of anaesthesia | 0.889 | ||
| Totally intravenous | 531 / 3919 (13.5) | 282 / 2144 (13.2) | |
| Volatile | 2763 / 3919 (70.5) | 1523 / 2144 (71.0) | |
| Balanced | 625 / 3919 (15.9) | 339 / 2144 (15.8) | |
| Use of NMBA | 3431 / 3919 (87.5) | 1878 / 2144 (87.6) | 0.959 |
| Desaturation | 146 / 3919 (3.7) | 85 / 2144 (4.0) | 0.641 |
| Unplanned recruitment manoeuvre | 140 / 3919 (3.6) | 68 / 2144 (3.2) | 0.412 |
| Hypotension | 1050 / 3919 (26.8) | 524 / 2144 (24.4) | 0.045 |
| Arrhythmia | 26 / 3919 (0.7) | 12/2144 (0.6) | 0.624 |
| Need of vasoactive drugs | 881 / 3919 (22.5) | 443 / 2144 (20.7) | 0.101 |
| Reversal of NMBA | 1584 /3919 (40.4) | 851 / 2144 (39.7) | 0.581 |
| Antibiotic prophylaxis | 2800 / 3919 (71.4) | 1524 / 2144 (71.1) | 0.764 |
| Mechanical ventilation characteristics | |||
| Tidal volume (ml/kg PBW) | 8.2 [7.3 to 9.2] | 8.2 [7.4 to 9.1] | 0.426 |
| PEEP (cmH2O) | 4 [0 to 5] | 3 [0 to 5] | 0.933 |
| Peak pressure (cmH2O) | 17 [15 to 21] | 17 [15 to 21] | 0.610 |
| FiO2 | 0.50 [0.45 to 0.70] | 0.50 [0.45 to 0.70] | 0.952 |
| Respiratory rate, bpm Clinical outcomes | 12 [12 to 13] | 12 [12 to 13] | 0.599 |
| PPC | 419 / 3919 (10.7) | 246 / 2144 (11.5) | 0.351 |
| Hospital length of stay (days) | 2 [1 to 4] | 2 [1 to 4] | 0.937 |
| In-hospital mortality | 23 / 3649 (0.6) | 9 / 1986 (0.5) | 0.397 |
Data are median [interquartile range] and No./Total (%). ARISCAT, Assess Respiratory Risk in Surgical Patients in Catalonia risk; ASA PS, American Society of Anaesthesiology physical score; BPM, breaths per minute; COPD, chronic obstructive pulmonary disease; FiO2, inspired fraction of oxygen; GI, gastrointestinal; HB, hepatobiliary; HN, head and neck; LQ, lower quartile; NMBA, neuromuscular blocking agents; PBW, predicted body weight; PEEP, positive end-expiratory pressure; PPC, postoperative pulmonary complication; SpO2, peripheral oxygen saturation; UQ, upper quartile.
Defined as haemoglobin < 10 g dl−1.
Neuromuscular disease affecting the respiratory system.
A patient can have more than one type of surgical procedure or technique.
Carotid endarterectomy, aortic surgery and peripheral vascular taken together.
Model development, validation, specification and performance
The results of the univariable logistic regression are summarised in eTable 5, http://links.lww.com/EJA/A162. Multivariable adjustment showed six patient characteristics [higher age, higher ASA physical score, pre-operative anaemia, pre-operative lower SpO2 and a history of active cancer or obstructive sleep apnoea, two procedure-related features (urgent/emergency surgery and longer duration of surgery) and five intra-operative events [use of an airway other than a supraglottic device, the use of intravenous anaesthetic agents along with volatile agents (balanced anaesthesia), intra-operative desaturation, higher levels of PEEP and use of vasopressors] to have an independent association with occurrence of PPCs (Table 2). Bootstrap validation indicated that all 13 factors were present in more than 80% of bootstrap samples and thus all were kept in the final model (eTable 6, http://links.lww.com/EJA/A162). The Hosmer-Lemeshow statistic was 6.626 (P = 0.578). The c-statistic of the model was 0.781 (95% CI, 0.758 to 0.804; P < 0.001) in the development cohort (Fig. 2a). In the validation cohort, the c-statistic was 0.724 (95% CI, 0.690 to 0.757; P < 0.001) and Hosmer-Lemeshow was 11.388 (P = 0.181) (Fig. 2a). The Brier score for the model in the validation cohort is 0.093. Calibration plots are shown in Fig. 2b and c. Considering the overall cohort, the LAS VEGAS score performed better than the ARISCAT score: AUC for LAS VEGAS score was 0.757 [95% CI, 0.746 to 0.776) vs. AUC for ARISCAT score 0.700 (95% CI, 0.678 to 0.711), P < 0.001] (Fig. 2d).
Table 2.
Multivariable logistic regression of risk factor for postoperative pulmonary complications in the development cohort
| β coefficient | Odds ratio (95% Cl) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 0.012 | 1.01 (1.00 to 1.02) | 0.004 |
| ASA PS | 0.290 | 1.34 (1.12 to 1.59) | 0.001 |
| Preoperative anaemia | 0.572 | 1.77 (1.10 to 2.85) | 0.018 |
| Preoperative SpO2 | −0.057 | 0.94 (0.90 to 0.99) | 0.021 |
| Cancer | 0.544 | 1.72 (1.18 to 2.52) | 0.005 |
| Obstructive sleep apnoea | 0.917 | 2.50 (1.40 to 4.47) | 0.002 |
| Surgical characteristics | |||
| Condition of surgery | |||
| Elective 1 (Reference) | 1 (Reference) | ||
| Urgency | 0.769 | 2.16 (1.54 to 3.02) | < 0.001 |
| Emergency | 0.941 | 2.56 (1.43 to 4.59) | 0.002 |
| Duration of surgery (min) | 0.005 | 1.00 (1.00 to 1.01) | < 0.001 |
| Intra-operative characteristics | |||
| Use of supraglottic device - | −0.653 | 0.52 (0.31 to 0.86) | 0.011 |
| Type of anaesthesia | |||
| Totally intravendu (Reference) | 1 (Reference) | ||
| Volatile | 0.002 | 1.00 (0.71 to 1.41) | 0.992 |
| Balanced | 0.590 | 1.80 (1.20 to 2.70) | 0.004 |
| Desaturation | 1.101 | 3.01 (1.99 to 4.54) | < 0.001 |
| Need of vasoactive drug | 0.405 | 1.50 (1.17 to 1.92) | 0.002 |
| Mechanical ventilation characteristics | |||
| PEEP (cmH2O) | 0.078 | 1.08 (1.03 to 1.13) | 0.002 |
c-index (95% CI): 0.781 (0.758 to 0.804) (P < 0.001). Hosmer-Lemeshow Chi-square test: 6.626 (P = 0.578). ASA PS, American Society of Anaesthesiology physical score; CI, confidence interval; PEEP, positive end-expiratory pressure; SpO2, peripheral oxygen saturation.
Fig. 2.
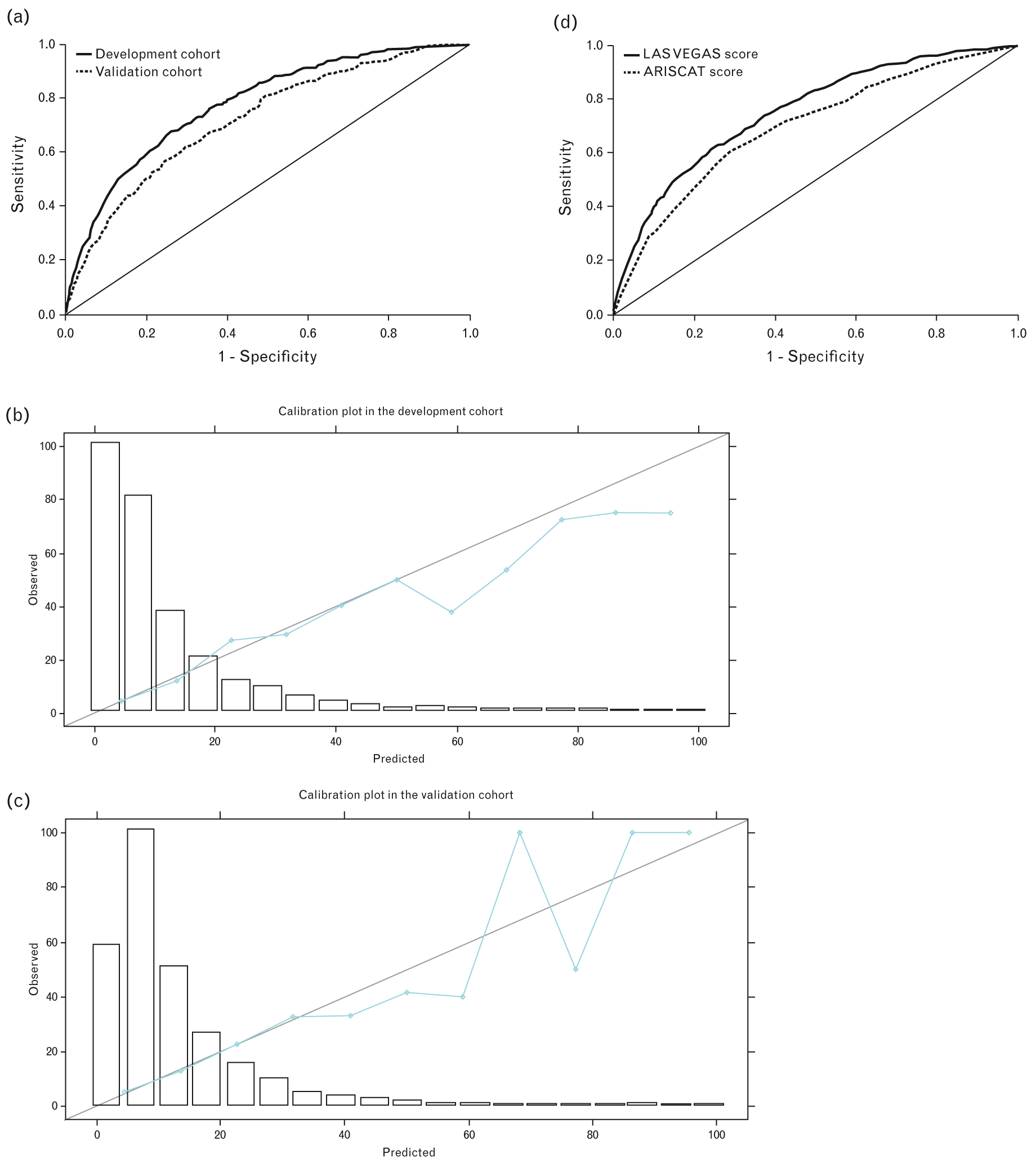
(a) Receiver operating characteristic (ROC) curve using β coefficients; (b) calibration plot in the development cohort; (c) calibration plot in the validation cohort; (d) comparison of the ROC curves of LAS VEGAS score and ARISCAT score in the overall cohort.
LAS VEGAS risk score for postoperative pulmonary complications
The simplified risk score is summarised in Table 3. The ROC curves for the simplified score in the development and in the validation cohort is shown in Fig. 3a. In the development cohort, the c-statistic was 0.778 (95% CI, 0.755 to 0.801; P < 0.001), and in the validation cohort, it was 0.703 (95% CI, 0.667 to 0.739; P < 0.001) (Fig. 3a). Considering the overall cohort, the simplified score performed better than the ARISCAT score: AUC for LAS VEGAS score: 0.750 (95% CI, 0.731 to 0.770) vs. AUC for ARISCAT score 0.700 (95% CI, 0.678 to 0.711), P < 0.001 (Fig. 3b). Categorisation using cutoffs of 7 and 17 produced three groups with clearly different incidences of PPCs (Fig. 4).
Table 3.
Multivariable logistic regression of risk factor for postoperative pulmonary complications in the development cohort (simplified risk score)
| β coefficient | Odds ratio (95% Cl) | Risk scorea | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | |||
| ≤ 46 | 1 (Reference) | 1 (Reference) | |
| 47–67 | 0.291 | 1.34 (0.99 to 1.81) | 3 |
| ≥ 68 | 0.407 | 1.50 (1.07 to 2.11) | 4 |
| ASA PS | |||
| < 3 | 1 (Reference) | 1 (Reference) | |
| ≥ 3 | 0.644 | 1.90 (1.49 to 2.44) | 6 |
| Preoperative anaemia | 0.483 | 1.62 (1.01 to 2.59) | 5 |
| Preoperative SpO2 | |||
| > 96 | 1 (Reference) | 1 (Reference) | |
| ≥ 96 | 0.249 | 1.28 (1.01 to 1.62) | 2 |
| Cancer | 0.538 | 1.71 (1.17 to 2.50) | 5 |
| Obstructive sleep apnoea | 0.900 | 2.46 (1.38 to 4.39) | 9 |
| Surgical characteristics Condition of surgery | |||
| Elective | 1 (Reference) | 1 (Reference) | |
| Urgency | 0.760 | 2.14 (1.53 to 2.98) | 8 |
| Emergency | 0.943 | 2.57 (1.45 to 4.55) | 9 |
| Duration of surgery (min) | |||
| ≤ 55 | 1 (Reference) | 1 (Reference) | |
| 56–134 | 0.390 | 1.48 (1.08 to 2.01) | 4 |
| ≥ 135 | 1.121 | 3.07 (2.23 to 4.21) | 11 |
| Intra-operative characteristics | |||
| Use of supraglottic device | −0.644 | 0.525 (0.31 to 0.87) | −6 |
| Type of anaesthesia | |||
| Totally intravenous | 1 (Reference) | 1 (Reference) | |
| Volatile | −0.010 | 0.99 (0.70 to 1.40) | 0 |
| Balanced | 0.535 | 1.71 (1.14 to 2.56) | 5 |
| Desaturation | 1.229 | 3.42 (2.29 to 5.10) | 12 |
| Need of vasoactive drug | 0.473 | 1.60 (1.25 to 2.05) | 5 |
| Mechanical ventilation characteristics | |||
| PEEP (cmH2O) | |||
| ≤ 2 | 1 (Reference) | 1 (Reference) | |
| 3–4 | 0.358 | 1.43 (1.05 to 1.95) | 3 |
| ≥ 5 | 0.400 | 1.49 (1.14 to 1.96) | 4 |
ASA PS, American Society of Anaesthesiology physical score; CI, confidence interval; PEEP, positive end-expiratory pressure; SpO2, peripheral oxygen saturation.
The simplified risk score was the sum of each β logistic regression coefficient multiplied by 10, after rounding off its value.c-index (95% CI): 0.778 (0.755 to 0.801) (P < 0.001 ).Hosmer-Lemeshow Chi-square test: 15.414 (P = 0.052).
Fig. 3.
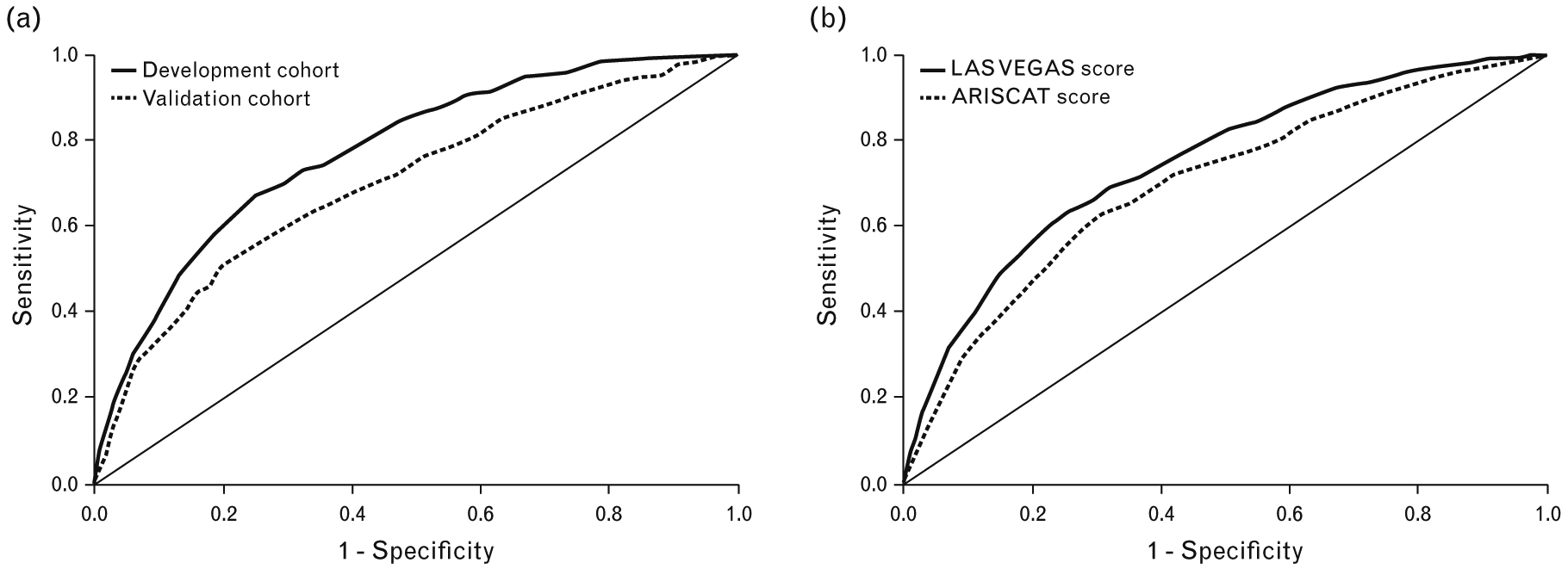
(a) Receiver operating characteristic (ROC) curve using the simplified score; (b) comparison of the ROC curves of simplified LAS VEGAS score and ARISCAT score in the overall cohort.
Fig. 4.
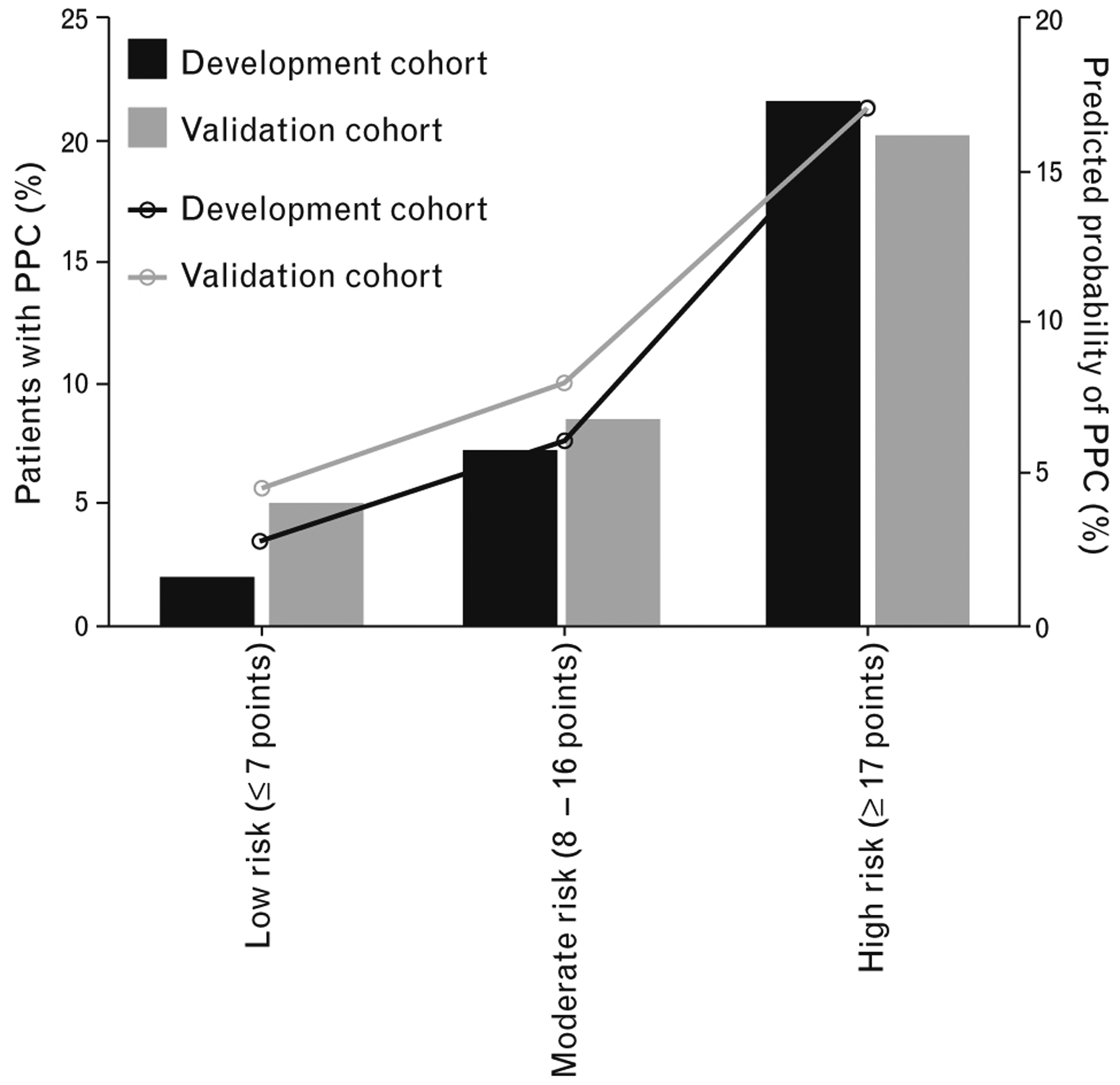
Incidence and predicted probability of PPCs according to cut-offs of simplified LAS VEGAS risk score. Low risk, ≤ 7; moderate risk, 8–16; and high risk, ≥17. PPCs, postoperative pulmonary complications.
The performance of the LAS VEGAS risk score in predicting severe PPCs when using the β coefficients as well as when using the simplified score is shown in eFigure 1, http://links.lww.com/EJA/A162. Categorisation using the same cutoffs as described above again produced three groups with clearly different incidences (eFigure 2, http://links.lww.com/EJA/A162). In all these analyses, the LAS VEGAS risk score performed better than the ARISCAT score (eFigure 1, http://links.lww.com/EJA/A162). Finally, the removal of PEEP from the model did not change the c-statistics: 0.721 (95% CI, 0.688 to 0.754) without PEEP vs. 0.724 (0.690 to 0.757) with PEEP, P = 0.530 (eFigure 3, http://links.lww.com/EJA/A162).
Discussion
In this secondary analysis of the LAS VEGAS study, 13 easily collected peri-operative characteristics had an independent association with the development of PPCs. The combination of these peri-operative characteristics into a predictive score resulted in a score with moderate discriminative power for identifying patients at risk of a PPC, as indicated by the AUC of the ROC curve.
The finding that age was an independent predictor of PPCs is in line with the existing literature.2,3,20–22 The ASA physical score was also a predictor of PPCs. In fact, the ASA physical score reflects comorbidity and functional capacity of patients and PPCs are related to target organ dysfunctions and the general health of patients.2,14,15 However, in a recent study, the ASA physical score by itself did not perform well in predicting PPCs after renal transplant.23 The association between pre-operative anaemia and the development of PPCs was previously shown in the ARISCAT score, as the association between pre-operative low SpO2 measurements and PPCs.2,3 A low SpO2 in room air may reflect a poor baseline cardiopulmonary status.2,3,5 Finally, the association between duration of surgery and the development of PPCs was also in accordance with the findings from ARISCAT.2 In the present analysis, unlike ARISCAT, the actual and not the predicted duration of surgery was used as a risk factor.
Obstructive sleep apnoea showed an important risk association with PPCs, which has not been shown before, but a recent investigation suggested that postoperative complications occurred at a higher rate in obstructive sleep apnoea patients who underwent hip or knee replacement.24 Such a finding could be explained partially by the combined effects of anaesthetic agents, sedatives and narcotics, which relax upper airway muscles and increase upper airway resistance, thus aggravating the effects of obstructive sleep apnoea.25 Also of interest, a history of active cancer increased the risk of PPCs. The reasons for this are not entirely clear. It may be the result of some disease process that is more frequent in cancer patients, such as pulmonary dysfunction due to mass effects, pleural effusions or metastasis, immunosuppression or general frailty.26
The association between intra-operative desaturations and development of PPCs could reflect higher pulmonary instability during intra-operative ventilation, most likely the occurrence of atelectasis and airway closure, and a consequent decrease in functional residual capacity. The association between intra-operative use of vasoactive drugs and development of PPCs could be the effect of more intensive mechanical ventilation strategies, for example the use of higher PEEP and recruitment manoeuvres, leading to a reduction in venous return, low cardiac output and hypotension.27,28 Another possible explanation is systemic inflammation leading to hypotension with subsequent organ dysfunction, including pulmonary dysfunction.29
The association between the use of supraglottic devices and PPCs may be due to the fact that these devices are used more frequently for patients who are considered to be more stable during surgery with a lower risk of perioperative complications,13,30 and for less complex or shorter-lasting surgical procedures.30 The same is true for PEEP, where its impact could be explained by the use of higher levels in more severely ill patients and in more complex situations. In addition, the relatively small range of PEEP used here may not reflect substantial physiological effects. Taken together, the data do not support the idea that a prospective general use of lower PEEP, especially in patients with the potential to benefit from it, will reduce the incidence of PPCs. Also, it should be noted that the level of PEEP used may not be a matter of perceived clinical risk or problems in gas exchange, but rather it is a general concept within a specific department. In line with this, the addition of the PEEP level to the model had no effect. Indeed, the area under the ROC-curves are close to identical. It is important to emphasise that some studies suggest a beneficial impact of PEEP in patients undergoing abdominal surgery31,32; nevertheless, more evidence is needed to confirm the impact of PEEP in this group of patients.
Notably, the LAS VEGAS risk score performed better than the highly regarded ARISCAT risk score. But it is important to understand the possible reasons for the lower predictive performance of the ARISCAT score in the present study compared with the original description and validation. Previous evaluations suggested that the score performs better in Western compared with Eastern countries, and better in Spain than in the rest of Europe.2,3 We speculate that as several centres in the LAS VEGAS were outside Europe, average surgical and anaesthetic practice may have differed slightly from the very first cohort. Also, the ARISCAT score seems to have a better predictive value for higher risk patients, but in the LAS VEGAS study, patients at lower risk of complications formed the majority. However, the moderate performance of our score suggests it is useful for screening in this heterogeneous patient population, independent from geographic distributions. Although several factors included in the ARISCAT risk score were also included in the LAS VEGAS risk score, the addition of some important factors, including intra-operative complications and obstructive sleep apnoea, had a significant impact on the final score and this could explain its better performance. Moreover, for the development of the LAS VEGAS risk score, a larger number of patients, and consequently a larger number of events, was used than for the development of the ARISCAT risk score. Overfitting the model was thus less likely, decreasing the chance of underestimation in the probability of events in low-risk patients, and overestimation in high risk patients.33 However, it is important to note that the ARISCAT risk score is a pre-operative score, while the LAS VEGAS risk score also considered intra-operative variables. Indeed, the ability of a score to predict an event is higher when you are closer to the event.34 Other available pre-operative scores, such as the ‘Predictors of Respiratory Insufficiency and Mortality (PRIM)’ and the ‘Score for Prediction of Postoperative Respiratory Complications (SPORC)’,35,36 were not addressed in the present article. Nevertheless, the PRIM score has a good discriminative ability to predict the need for mechanical ventilation and in-hospital mortality in patients with acute cervical spine injury,35 and the SPORC also has a good discriminative power to predict PPCs in patients undergoing surgery.36
It is important to emphasise that the LAS VEGAS risk score should always be used in its total form and not be used to consider the impact of only one or two variables. For example, one should not focus on PEEP as a predictor of PPCs, once it is included in an analysis involving several other factors. The weight and relative importance of each factor in the final score was calculated in the presence of other factors, so it is of importance always to consider the whole score and not individual components.
The strengths of this analysis lie in the use of data from a broad surgical population in a prospective, international multicentre study. A fully representative target population extract was used, and a robust multivariable logistic regression method was applied allowing an appropriate validation in the present cohort.
Nevertheless, the present study has a number of limitations. First, the willingness of participating centres to join the study may have caused a selection bias. Second, any prospective observational study can interfere with daily practice. Third, there is always a possibility of unknown confounding factors. Fourth, we had no restriction on the number of centres per country, and this resulted in overrepresentation of some countries. Fifth, it is possible that additional variables that improve the prediction may be identified in future studies and need to be added to the LAS VEGAS risk score. Sixth, it is important to note that our definition of PPCs differs from that used in other studies.2,3,23 We chose to follow the approach of most PPCs studies to date, in which risk is established for a composite outcome, and that composite outcome can be attained by the presence of one or several of the list of complications. Seventh, the discriminative power was moderate. Finally, despite the use of advanced statistical methods, observational data typically cannot elicit complex aetiological relationships.
Conclusion
The LAS VEGAS score is a simple risk score, with moderate discriminative performance, for predicting PPCs and is based on 13 easy to capture peri-operative characteristics. It could be useful for identifying individual patients at a high risk of PPCs, and in the design of future trials to assess interventions to prevent these complications. However, external validation is still needed to confirm the accuracy of the score.
Supplementary Material
Acknowledgements relating to this article
The LAS VEGAS Study Writing Committee for this secondary analysis: Ary Serpa Neto (Hospital Israelita Albert Einstein, São Paulo, Brazil); Luiz Guilherme Villares da Costa (Hospital Israelita Albert Einstein, São Paulo, Brazil); Sabrine N.T. Hemmes (Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands); Marcelo Gama de Abreu (University Hospital Dresden, Technische Universität Dresden, Dresden, Germany); Paolo Pelosi (Ospedale Policlinico San Martino - IRCCS per l’Oncologia, University of Genoa, Genoa, Italy); and Marcus J. Schultz (Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands).
Financial support and sponsorship: the LAS VEGAS study was endorsed and partly funded by a research grant from the European Society of Anaesthesiology through their Clinical Trial Network. MFVM was supported by NIH-NHLBI Grant 1R34HL123438.
Footnotes
LAS VEGAS (Local Assessment of Ventilatory Management During General Anaesthesia for Surgery). Members of the LAS VEGAS writing committee and the LAS VEGAS Steering committee are listed at the end of this article.
The LAS VEGAS Study Steering Committee: Sabrine N.T. Hemmes (Academic Medical Center, Amsterdam, the Netherlands); Ary Serpa Neto (Hospital Israelita Albert Einstein; São Paulo, Brazil); Jaume Canet (Hospital Universitari Germans Trias I Pujol, Barcelona, Spain); Göran Hedenstierna (University Hospital Uppsula, Uppsala, Sweden); Samir Jaber (Saint Eloi University Hospital, Montpellier, France); Michael Hiesmayr (Medical University Vienna, Vienna, Austria); Markus W. Hollmann (Academic Medical Center, Amsterdam, the Netherlands); Gary H. Mills (Sheffield Teaching Hospitals, Sheffield, U.K.); Marcos F. Vidal Melo (Massachusetts General Hospital, Boston, U.S.A.); Rupert Pearse (Queen Mary University of London, London, U.K.); Christian Putensen (University Hospital Bonn, Bonn, Germany); Werner Schmid (Medical University Vienna, Vienna, Austria); Paolo Severgnini (University of Insubria, Varese, Italy); Hermann Wrigge (University of Leipzig, Leipzig, Germany); Marcelo Gama de Abreu (University Hospital Dresden, Dresden, Germany); Paolo Pelosi (Ospedale Policlinico San Martino - IRCCS per l’Oncologia, University of Genoa, Genoa, Italy); and Marcus J. Schultz (Academic Medical Center, Amsterdam, the Netherlands).
The LAS VEGAS study collaborators are listed in the Supplemental digital content, http://links.lww.com/EJA/A163.
Assistance with the study: we are indebted to all participating research nurses, nurse anaesthetists, surgeons, other physicians and our patients. We particularly wish to acknowledge Brigitte Leva, Sandrine Damster and Benoit Plichon from the Clinical Trial Network of the European Society of Anaesthesiology for their expertise and professional help in coordinating the study and cleaning the data for the LAS VEGAS study. Without the aforementioned, the LAS VEGAS study would not have been possible. All members of the Steering Committee contributed to the design and conduct of the LAS VEGAS study.
Conflicts of interest: none.
Presentation: the study was presented in part at Euroanaesthesia 2017.
Contributor Information
Ary Serpa Neto, Hospital Israelita Albert Einstein, São Paulo, Brazil.
Luiz Guilherme V. da Costa, Hospital Israelita Albert Einstein, São Paulo, Brazil
Sabrine N.T. Hemmes, Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands
Jaume Canet, Hospital Universitari Germans Trias I Pujol, Barcelona, Spain.
Göran Hedenstierna, University Hospital Uppsula, Uppsala, Sweden.
Samir Jaber, Saint Eloi University Hospital, Montpellier, France.
Michael Hiesmayr, Medical University Vienna, Vienna, Austria.
Markus W. Hollmann, Academic Medical Center, Amsterdam, the Netherlands
Gary H. Mills, Sheffield Teaching Hospitals, Sheffield, UK
Marcos F. Vidal Melo, Massachusetts General Hospital, Boston, USA
Rupert Pearse, Queen Mary University of London, London, UK.
Christian Putensen, University Hospital Bonn, Bonn, Germany.
Werner Schmid, Medical University Vienna, Vienna, Austria.
Paolo Severgnini, University of Insubria, Varese, Italy.
Hermann Wrigge, University of Leipzig, Leipzig, Germany.
Marcelo Gama de Abreu, University Hospital Dresden, Dresden, Germany.
Paolo Pelosi, Ospedale Policlinico San Martino - IRCCS per I’Oncologia, University of Genoa, Genoa, Italy.
Marcus J. Schultz, Academic Medical Center, Amsterdam, the Netherlands
References
- 1.Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet 2012; 380:1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010; 113:1338–1350. [DOI] [PubMed] [Google Scholar]
- 3.Mazo V, Sabate S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology 2014; 121:219–231. [DOI] [PubMed] [Google Scholar]
- 4.Serpa Neto A, Hemmes SN, Barbas CS, et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med 2014; 2:1007–1015. [DOI] [PubMed] [Google Scholar]
- 5.Kor DJ, Warner DO, Alsara A, et al. Derivation and diagnostic accuracy of the surgical lung injury prediction model. Anesthesiology 2011; 115: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kor DJ, Lingineni RK, Gajic O, et al. Predicting risk of postoperative lung injury in high-risk surgical patients: a multicenter cohort study. Anesthesiology 2014; 120:1168–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serpa Neto A, Hemmes SN, Barbas CS, et al. Protective versus conventional ventilation for surgery: a systematic review and individual patient data meta-analysis. Anesthesiology 2015; 123:66–78. [DOI] [PubMed] [Google Scholar]
- 8.Lamarche Y, Elmi-Sarabi M, Ding L, et al. A score to estimate 30-day mortality after intensive care admission after cardiac surgery. J Thorac Cardiovasc Surg 2017; 153:1118–1125. [DOI] [PubMed] [Google Scholar]
- 9.Silva JM Jr, de Oliveira AM, Nogueira FA, et al. The effect of excess fluid balance on the mortality rate of surgical patients: a multicenter prospective study. Crit Care 2013; 17:R288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riss S, Mittlböck M, Riss K, et al. Intraoperative complications have a negative impact on postoperative outcomes after rectal cancer surgery. Int J Surg 2014; 12:833–836. [DOI] [PubMed] [Google Scholar]
- 11.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. J Clin Epidemiol 2015; 68:134–143. [DOI] [PubMed] [Google Scholar]
- 12.Hemmes SN, de Abreu MG, Pelosi P, Schultz MJ. ESA Clinical Trials Network 2012: LAS VEGAS-local assessment of ventilatory management during general anaesthesia for surgery and its effects on postoperative pulmonary complications: a prospective, observational, international, multicentre cohort study. Eur J Anaesthesiol 2013; 30:205–207. [DOI] [PubMed] [Google Scholar]
- 13.LAS VEGAS Investigators. Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications - LAS VEGAS, an observational study in 29 countries. Eur J Anesthesiol 2017; 34:492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smetana GW, Lawrence VA, Cornell JE. American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med 2006; 144:581–595. [DOI] [PubMed] [Google Scholar]
- 15.McAlister FA, Bertsch K, Man J, et al. Incidence of and risk factors for pulmonary complications after nonthoracic surgery. Am J Respir Crit Care Med 2005; 171:514–517. [DOI] [PubMed] [Google Scholar]
- 16.McCullagh P, Nelder JA. Generalized linear models. 2nd ed. (Monographs on Statistics and Probability 37; ). London: Chapman & Hall; 1989. [Google Scholar]
- 17.Alba AC, Agoritsas T, Walsh M, et al. Discrimination and calibration of clinical prediction models: users’ guides to the medical literature. JAMA 2017; 318:1377–1384. [DOI] [PubMed] [Google Scholar]
- 18.Henderson AR. The bootstrap: a technique for data-driven statistics. Using computer-intensive analyses to explore experimental data. Clin Chim Acta 2005; 359:1–26. [DOI] [PubMed] [Google Scholar]
- 19.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44:837–845. [PubMed] [Google Scholar]
- 20.Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg 2006; 203:865–877. [DOI] [PubMed] [Google Scholar]
- 21.Utsumi M, Shimizu J, Miyamoto A, et al. Age as an independent risk factor for surgical site infections in a large gastrointestinal surgery cohort in Japan. J Hosp Infect 2010; 75:183–187. [DOI] [PubMed] [Google Scholar]
- 22.Berry AJ, Smith RB 3rd, Weintraub WS, et al. Age versus comorbidities as risk factors for complications after elective abdominal aortic reconstructive surgery. J Vasc Surg 2001; 33:345–352. [DOI] [PubMed] [Google Scholar]
- 23.Kupeli E, Er Dedekarginoglu B, Ulubay G, et al. American Society of Anesthesiologists classification versus ARISCAT Risk Index: predicting pulmonary complications following renal transplant. Exp Clin Transplant 2017; 15:208–213. [DOI] [PubMed] [Google Scholar]
- 24.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc 2001; 76:897–905. [DOI] [PubMed] [Google Scholar]
- 25.Boushra NN. Anaesthetic management of patients with sleep apnoea syndrome. Can J Anaesth 1996; 43:599–616. [DOI] [PubMed] [Google Scholar]
- 26.Miyakita H, Sadahiro S, Saito G, et al. Risk scores as useful predictors of perioperative complications in patients with rectal cancer who received radical surgery. Int J Clin Oncol 2017; 22:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology Hemmes SN, Gama de Abreu M, Pelosi P, et al. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014; 384:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funk DJ, Jacobsohn E, Kumar A. Role of the venous return in critical illness and shock: part II-shock and mechanical ventilation. Crit Care Med 2013; 41:573–579. [DOI] [PubMed] [Google Scholar]
- 29.Luecke T, Pelosi P. Clinical review: positive end-expiratory pressure and cardiac output. Crit Care 2005; 9:607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramachandran SK, Kumar AM. Supraglottic airway devices. Respir Care 2014; 59:920–932. [DOI] [PubMed] [Google Scholar]
- 31.de Jong MAC, Ladha KS, Vidal Melo MF, et al. Differential effects of intraoperative positive end-expiratory pressure (PEEP) on respiratory outcome in major abdominal surgery versus craniotomy. Ann Surg 2016; 264:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ladha K, Vidal Melo MF, McLean DJ, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 2015; 351:h3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavlou M, Ambler G, Seaman SR, et al. How to develop a more accurate risk prediction model when there are few events. BMJ 2015; 351:h3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazo V, Sabaté S, Canet J. A race against time: planning postoperative critical care. Anesthesiology 2013; 119:498–500. [DOI] [PubMed] [Google Scholar]
- 35.Durga P, Sahu BP, Mantha S, Ramachandran G. Development and validation of predictors of respiratory insufficiency and mortality scores: simple bedside additive scores for prediction of ventilation and in-hospital mortality in acute cervical spine injury. Anesth Analg 2010; 110:134–140. [DOI] [PubMed] [Google Scholar]
- 36.Brueckmann B, Villa-Uribe JL, Bateman BT, et al. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology 2013; 118:1276–1285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


