Figure 4. Enhancing RGS6 levels in adult-born neurons promotes neuronal maturation.
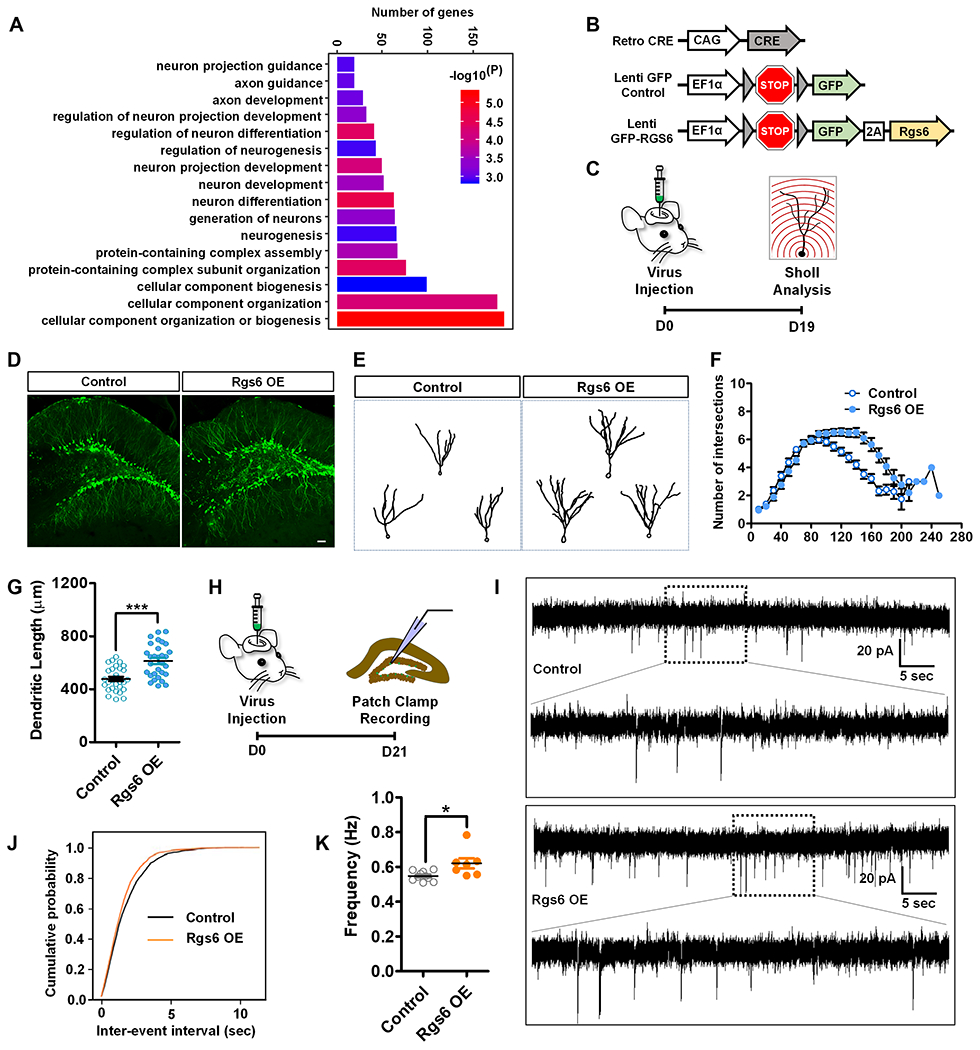
(A) GO analysis of Rgs6 (M5) module genes.
(B) A schematic illustration of viral vectors used for in vivo targeting of adult new neurons: retroviral vector expressing Cre recombinase (Retro-CRE), lentiviral vector with CRE-dependent expression of GFP (Lenti-GFP-Control), and lentiviral vector with CRE-dependent expression of GFP-RGS6 (Lenti-GFP-RGS6).
(C) Timeline of in vivo viral targeting for morphological analysis of adult-born new neurons.
(D) Sample confocal images of virus-infected adult new neurons. Scale bar, 50μm.
(E) Sample traces of GFP-expressing neurons.
(F) Overexpression of RGS6 increases the dendritic complexity compared to controls (F1,60= 26.357, p < 0.001, MANOVA).
(G) Exogenous expression of RGS6 increases dendritic length of adult new neurons compared to controls (p<0.0001, t-test).
(H) Timeline of in vivo viral targeting for electrophysiological analysis of adult-born new neurons.
(I) Representative traces of mEPSCs recorded from GFP+ new neurons in acute hippocampal slices derived from Control and RGS6 OE mice 3 weeks after retroviral injection.
(J-K) Cumulative probability distribution (Control, n= 10 cells; RGS6 OE, n= 7 cells.) and average frequency (P=0.0154, on two tailed t-test). *P< 0.05, **P< 0.01, ***P< 0.001.
