The current coronavirus disease 2019 (COVID-19) pandemic induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has already caused a global increase in hospitalizations and deaths. Unfortunately, effective medicines to fight this disease, especially in the severely ill patients, are still lacking [1]. Tocilizumab, a humanized monoclonal antibody used in rheumatoid arthritis treatment, might also be effective in treating severe COVID-19 as it could selectively target the interleukin-6 (IL-6) receptor [2]. Considering the uncertain efficacy of tocilizumab treatment in severe COVID-19, we conducted a systematic review and meta-analysis to clarify this added effect of tocilizumab.
We performed a systematic search of PubMed, Embase, Medline, Cochrane, and CNKI database through 25 July 2020, using the following search terms alone or in combination: (1) “COVID-19,” (2) “coronavirus,” (3) “SARS-CoV-2,” (4) “COVID,” (5) “anti-interleukin-6 receptor antibodies,” (6) “anti-IL-6 receptor antibodies,” (7) “anti-IL-6,” (8) “tocilizumab,” (9)“sarilumab,” and (10) “siltuximab.” Clinical trials regarding tocilizumab as a therapeutic intervention were selected. Two independent investigators selected eligible trials and extracted data from articles. Discrepancies in screening/data extraction were addressed by group discussion. Proportional variables were measured by odds ratio (OR) and corresponding 95% confidence intervals (CI). P values < 0.05 were considered statistically significant. Significant heterogeneity (P < 0.10 or I2 ≥ 50%) was evaluated by chi-square and I2 tests in a fixed-effect model. The comparison of the outcome between tocilizumab and control was conducted by using Review Manager 5.4 (Revman, The Cochrane Collaboration, Oxford, UK).
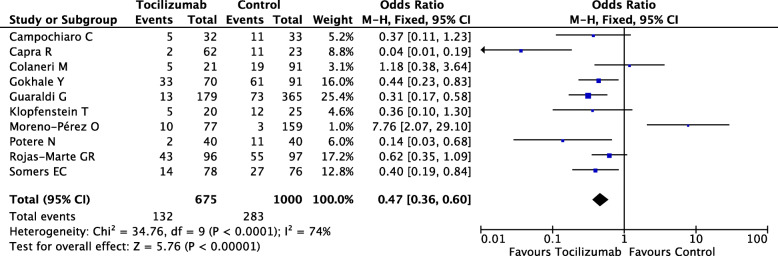
Finally, 10 studies involving 1675 severe COVID-19 patients were included, among which only one trial was a randomized controlled trial, while the rest were all retrospective cohort studies. These studies included COVID-19 patients who were older/elderly (mean/median age ≥ 52 years) in America, Europe, and India, among whom 675 patients received tocilizumab, while 1000 patients underwent standard care. Severe COVID-19 patients received tocilizumab via intravenous or subcutaneous formulation, while doses and administration time points varied. Standard care included hydroxychloroquine, lopinavir/ritonavir, remdesivir, azithromycin, low weight heparin, and/or methylprednisolone, among others (Table 1). Our meta-analysis result revealed a significant difference in mortality between tocilizumab group (132/675, 19.5%) and control group (283/1000, 28.3%) in the fixed-effect model (OR, 0.47; 95%Cl, 0.36–0.60; P < 0.00001), suggesting efficacy of tocilizumab treatment for severe COVID-19. However, high heterogeneity was also observed (I2 = 74%, P < 0.0001) as shown in Fig. 1. SARS-CoV-2 infection might cause a hyperimmune response associated with acute respiratory distress (ARDS), the latteris a leading cause of death for severe COVID-19 [3]. Uncontrolled immune activation would result in cytokine storm, also known as cytokine release syndrome (CRS), appearing as overproduction of pro-inflammatory cytokines and chemokines [4]. Severe COVID-19 patients always present elevated inflammatory markers, among which the elevation of IL-6 is associated with severity of COVID-19 [5]. Besides, the upregulated expression of IL-6 receptor (IL-6R) was also detected in COVID-19 patients [6]. Therefore, IL-6/IL6R might serve as a messenger not only for transmitting inflammatory signals throughout the lung and other organs but also by activating cellular signal pathway, thus causing ARDS and multiple organ dysfunction. It is reasonable to speculate that IL-6 blockade is beneficial for avoiding poor prognosis.
Table 1.
Study characteristics and demographics of included severely ill coronavirus disease 2019 (COVID-19) patients
| Article | Study design | Country | Total patients | Mean/median age (years) | Standard care | Tocilizumab treatment | Patients category | Primary outcomes |
|---|---|---|---|---|---|---|---|---|
|
Campochiaro C Eur J Intern Med 2020 |
Single-center retrospective cohort study | Italy | 65 |
60 (control) 64 (tocilizumab) |
Hydroxychloroquine, lopinavir/ritonavir, ceftriaxone, azithromycin | First intravenous 400 mg, second 400 mg was administered due to progressive respiratory worsening | Severe COVID-19 patients with hyper-inflammatory features admitted outside ICU requiring NIV and/or high-flow supplemental O2 | Safety, efficacy |
|
Capra R Eur J Intern Med 2020 |
Retrospective observational study | Italy | 85 |
70 (control) 63 (tocilizumab) |
Hydroxychloroquine, lopinavir/ritonavir | Tocilizumab once within 4 days | COVID-19-related pneumonia and respiratory failure, not needing mechanical ventilation | Survival rate |
|
Colaneri M Microorganisms 2020 |
Retrospective case-control study | Italy | 112 |
64 (control) 62 (tocilizumab) |
Hydroxychloroquine, azithromycin, low weight heparin, methylprednisolone | First administration was 8 mg/kg (up to a maximum 800 mg per dose) intravenously, repeated after 12 h | Critically ill patients with severe COVID-19 pneumonia | Admission to the ICU and 7-day mortality rate |
|
Gokhale Y EClinicalMedicine 2020 |
Retrospective cohort study | India | 161 |
55 (control) 52 (tocilizumab) |
Antibiotics, hydroxychloroquine oseltamivir, low molecular weight heparin, methylprednisolone | A single intravenous dose of 400 mg | COVID-19 with oxygen saturation of 94% or less despite giving supplemental oxygen of 15 L/min via non-rebreathing mask or PaO2/FiO2 ratio of less than 200 | Death |
|
Guaraldi G Lancet Rheumatol 2020 |
Retrospective observational cohort study | Italy | 544 |
69 (control) 64 (tocilizumab) |
Oxygen supply to target SaO2 reaching at least 90%, hydroxychloroquine, azithromycin at the physician’s discretion when suspecting a bacterial respiratory super-infection, lopinavir–ritonavir or darunavir–cobicistat, low molecular weight heparin | Intravenous tocilizumab was administered at 8 mg/kg bodyweight (up to a maximum of 800 mg) administered twice, 12 h apart; the subcutaneous formulation was used when there was a shortage of the intravenous formulation, at a dose of 162 mg administered in two simultaneous doses, one in each thigh | Severe pneumonia defined at least one of the following: presence of a respiratory rate of 30 or more breaths per minute, peripheral blood SaO2 of less than 93% in room air, a ratio of PaO2 to FiO2 of less than 300 mmHg in room air, and lung infiltrates of more than 50% within 24–48 h, according to Chinese management guidelines for COVID-19 | Death or invasive mechanical ventilation |
|
Klopfenstein T Med Mal Infect 2020 |
Retrospective case-control study | France | 45 |
71 (control) 77 (tocilizumab) |
Hydroxychloroquine or lopinavir-ritonavir, antibiotics, less commonly corticosteroids | 1 or 2 doses (no detail was reported) | All critically COVID-19 patients in tocilizumab group, fewer critically ill patients in control | Death and/or ICU admissions |
|
Moreno-Pérez O J Autoimmun 2020 |
Retrospective cohort study | Spain | 236 |
57 (control) 62 (tocilizumab) |
No detail was reported | Initial 600 mg, with a second or third dose (400 mg) in case of persistent or progressive disease | Severe COVID-19 pneumonia | All-cause mortality |
|
Potere N Ann Rheum Dis 2020 |
Retrospective case–control study | Italy | 80 |
54 (control) 56 (tocilizumab) |
Hydroxychloroquine, darunavir/cobicistat, lopinavir/ritonavir, systemic corticosteroid | 324 mg given as two concomitant subcutaneous injections | Severe COVID-19 pneumonia with hypoxemia (oxygen saturation < 90% on room air) requiring supplemental oxygen through nasal cannulas or mask | Requirement of IMV or death |
| Rojas-Marte GR QJM: An International Journal of Medicine 2020 | Retrospective, case–control, single-center study | USA | 193 |
62 (control) 59 (tocilizumab) |
Hydroxychloroquine, azithromycin, corticosteroids anticoagulation, remdesivir, antibiotics for suspected bacterial infections, vasopressors | No detail was reported | Adult patients hospitalized with severe COVID-19 | Overall mortality rate |
|
Somers EC Clin Infect Dis 2020 |
Randomized controlled trial | USA | 154 |
60 (control) 55 (tocilizumab) |
Hydroxychloroquine, remdesivir, NSAIDs, ACEI/ARB, vasopressors, anticoagulation corticosteroid | The standard tocilizumab dose was 8 mg/kg (maximum 800 mg) × 1, additional doses were discouraged | Severe COVID-19 patients requiring mechanical ventilation | Survival probability after intubation |
Fig. 1.
Forest plot of pooled mortality of severely ill coronavirus disease 2019 (COVID-19) patients from included studies
Our meta-analysis had several limitations: (1) most included studies were retrospective analysis of cases, resulting in poor quality of the included studies; (2) the uniformity of the diagnostic criteria for severe COVID-19 needs to be improved, and the extraction of related factors is limited; and (3) extraction of the original data is incomplete, and some data cannot be converted due to the lack of relevant data.
In summary, this is the first meta-analysis demonstrating the efficacy of tocilizumab treatment in severely ill COVID-19 patients.
Acknowledgements
Not applicable.
Abbreviations
- ACEI
Angiotension converting enzyme inhibitors
- ARB
Angiotension receptors blockers
- ARDS
Acute respiratory distress syndrome
- COVID-19
Coronavirus disease 2019
- CI
Confidence intervals
- CRS
Cytokine release syndrome
- FiO2
Fraction of inspired oxygen
- ICU
Intensive care unit
- IL-6
Interleukin-6
- IMV
Invasive mechanical ventilation
- NSAID
Non-steroidal anti-inflammatory drugs
- NIV
Non- invasive Ventilation
- OR
Odds ratio
- PaO2
Partial pressure of oxygen
- SaO2
Oxygen saturation
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
Authors’ contributions
TBP contributed the conception and design of this review; ZJ wrote the paper. TBP and CW revised and edited this manuscript. All authors reviewed the draft and approved the final manuscript for submission.
Funding
This work was supported by the Zhejiang Provinicial Natural Science Foundation of China (LY20H010002) and the Medical and Health Research Program of Zhejiang Province (2019RC181) to TBP.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reddy SG. Population health, economics and ethics in the age of COVID-19. BMJ Glob Health. 2020;5. 10.1136/bmjgh-2020-003259. [DOI] [PMC free article] [PubMed]
- 2.Cully M. Immune status could determine efficacy of COVID-19 therapies. Nat Rev Drug Discov. 2020;19:431–434. doi: 10.1038/d41573-020-00110-3. [DOI] [PubMed] [Google Scholar]
- 3.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020. 10.1001/jama.2020.12839. [DOI] [PubMed]
- 4.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koutsakos M, Kedzierska K. A race to determine what drives COVID-19 severity. Nature. 2020;583:366–368. doi: 10.1038/d41586-020-01915-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhu LN, Yang PH, Zhao YZ, et al. Single-cell sequencing of peripheral blood mononuclear cells reveals distinct immune response landscapes of COVID-19 and influenza patients. Immunity. 2020. 10.1016/j.immuni.2020.07.009. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.