Fig 4.
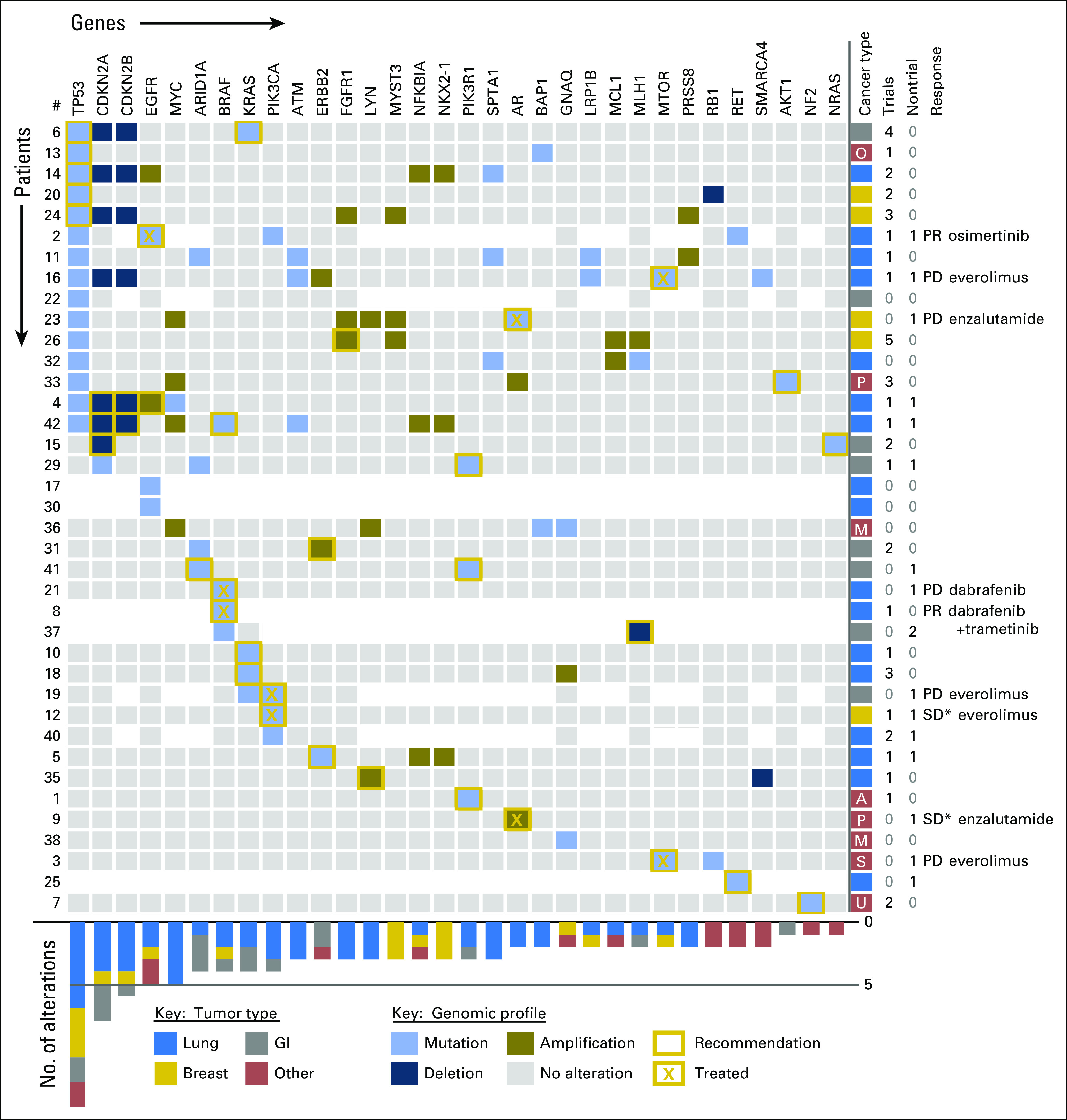
Genomic profiles, recommendations, treatment, and outcomes of the evaluated subjects. Genomic profiles are displayed for the 38 evaluable subjects. The genes with identified alterations are displayed as columns, with rows displaying the tumor profile for a given subject. Areas shown in white indicate genes that were not evaluated in that tumor. Gold boxes indicate genes that were considered actionable for targeted therapy, including clinical trials. An X within the box illustrates the accepted treatment recommendation. Cancer type by color code is shown on the right, as are the number of clinical trials and nontrial treatments recommended by the Precision Medicine Molecular Tumor Board, the response to therapy, and the treatment given. The chart on the bottom displays the frequency of alterations in each gene shown at the top by tumor type. Response Evaluation Criteria in Solid Tumors (RECIST 1.1) for measurable disease: PD, progressive disease; PR, partial response; SD, stable disease. (*) Nonmeasurable disease. A, adenoid cystic; M, melanoma; O, papillary serous ovary; P, prostate; S, alveolar rhabdomyosarcoma; U, carcinoma of unknown primary.
