FIG 2.
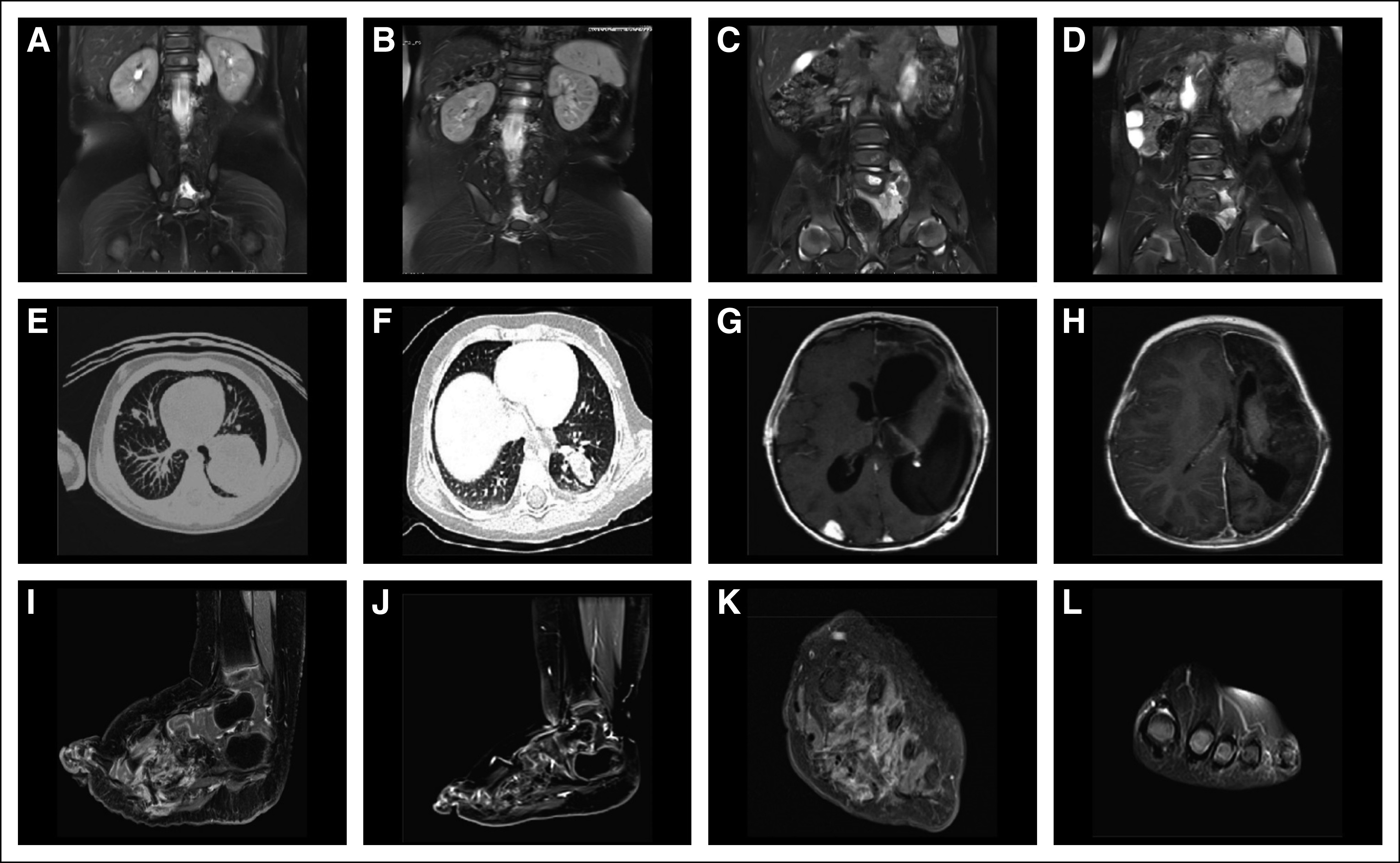
Selpercatinib activity in patients with soft-tissue sarcoma. Patient 3: (A, C) computed tomography (CT) scans of the abdomen at baseline and (B, D) after 6 months of treatment with selpercatinib, revealing multiple paraspinal retroperitoneal and pelvic lesions in a patient with infantile myofibroma/hemangiopericytoma harboring an MYH10-RET fusion. A partial response was observed after one cycle of selpercatinib; after six cycles, the paraspinal lesion had completely resolved, and the patient regained lower extremity neurologic function. Patient 4: CT scans at baseline of (E) the lungs and (G) brain and after 8 months of treatment with selpercatinib of (F) the lungs and (H) brain in a patient with an SPECC1L-RET fusion–positive congenital mesoblastic nephroma and infantile fibrosarcoma. After two cycles of selpercatinib, a partial response was observed with a 41% tumor reduction, which deepened to 66% by cycle 8. Patient 5: CT scans (I, K) at baseline and (J, L) after 2 months of treatment with selpercatinib of the left foot in a patient with an NCOA4-RET fusion–positive lipofibromatosis. Selpercatinib treatment resulted in a significant decrease in tumor burden leading to improvements in gait and locomotion.
