Abstract
Background:
The opticocarotid triangle (OCT) and the carotico-oculomotor triangle (COT) are two anatomical triangles used in accessing the interpeduncular region. Our objective is to evaluate if the anterior incisural width (AIW) is an indicator to predict the intraoperative exposure through both triangles.
Methods:
Twenty sides of 10 cadaveric heads were dissected and analyzed. The heads were divided into the following: Group A – narrow anterior incisura and Group B – wide anterior incisura – using 26.6 mm as a cutoff distance of the AIW. Subsequently, the area of the COT and the OCT in the transsylvian approach was measured, along with the maximum widths through the two trajectories in modified superior transcavernous approach.
Results:
The COT in the wide group was shown to have a significantly larger area compared with the COT in the narrow group (38.4 ± 12.64 vs. 58.3 ± 15.72 mm, P < 0.01). No difference between the two groups was reported in terms of the area of the OCT (50.9 ± 19.22 mm vs. 63.5 ± 15.53 mm, P = 0.20), the maximum width of the OCT (6.6 ± 1.89 vs. 6.5 ± 1.38 mm, P = 1.00), or the maximum width of the COT (11.7 ± 2.06 vs. 12.2 ± 2.32 mm, P = 0.50). Clinical cases were included.
Conclusion:
An AIW <26.6 mm is an unfavorable factor related to a limited COT area in a transsylvian approach for pathologies at the interpeduncular fossa. Preoperative identification and measurement of a narrow AIW can suggest the need to add a transcavernous approach.
Keywords: Anterior incisural width, Carotico-oculomotor triangle, Opticocarotid triangle

INTRODUCTION
The opticocarotid triangle (OCT)[7] and the carotico-oculomotor triangle (COT)[16] are two common anatomical triangles employed to approach a variety of lesions in the prepontine, crural, and interpeduncular cisterns through the standard pterional or the pretemporal transcavernous approach (PTA). The PTA offers a wide surgical corridor that can only be afforded by exposing the lateral wall of the cavernous sinus, drilling the anterior clinoid process extradurally, dividing the distal dural ring, and fully mobilizing the oculomotor nerve, including often drilling the posterior clinoid process.[9,14] The transsylvian exposure obtained by the pterional approach may not be sufficient to approach some ventrally and caudally positioned lesions as the triangles used in this approach can be narrow due to the variable configurations of the relevant neurovascular structures.[9]
In the tentorial incisura, the junction of the anterior petroclinoid ligament and the posterior petroclinoid ligament (JAPPL) is an identifiable landmark on MRI (T1WI, T2WI, or fast imaging employing steady-state acquisition), along with being a less ideal visualization on CTA. The distance between the JAPPL on both sides represents the anterior incisural width (AIW).[1]
We conducted a quantitative study to investigate the relationship of the AIW and the areas of the OCT and COT, which can be helpful in evaluating the exposure acquired through either triangle in addition to determining if a transcavernous approach should be selected.
MATERIALS AND METHODS
Surgical approaches
Twenty sides of 10 embalmed cadaveric heads were investigated. The head was fixed on a Mayfield head holder, turned about 30° laterally and tilted about 15° toward the floor, keeping the malar eminence at the highest point. A standard orbitozygomatic craniotomy was performed with the lateral wall and the roof of the orbit removed [Figure 1a-h].[2,15,18] The dura was peeled from the middle skull base; the anterior clinoid process and the superior orbital fissure were exposed. An extradural anterior clinoidectomy was performed after unroofing the optic canal and incising the frontotemporal dural fold.[5,10] The clinoid segment of the internal carotid artery (ICA) was identified [Figure 1i-k]. The dura was subsequently opened, the Sylvian fissure was fully dissected, and the OCT and the COT were identified [Figure 1l]. Furthermore, the distal dural ring was dissected and the clinoid segment of the carotid artery was exposed through an intradural perspective [Figure 1m].
Figure 1:
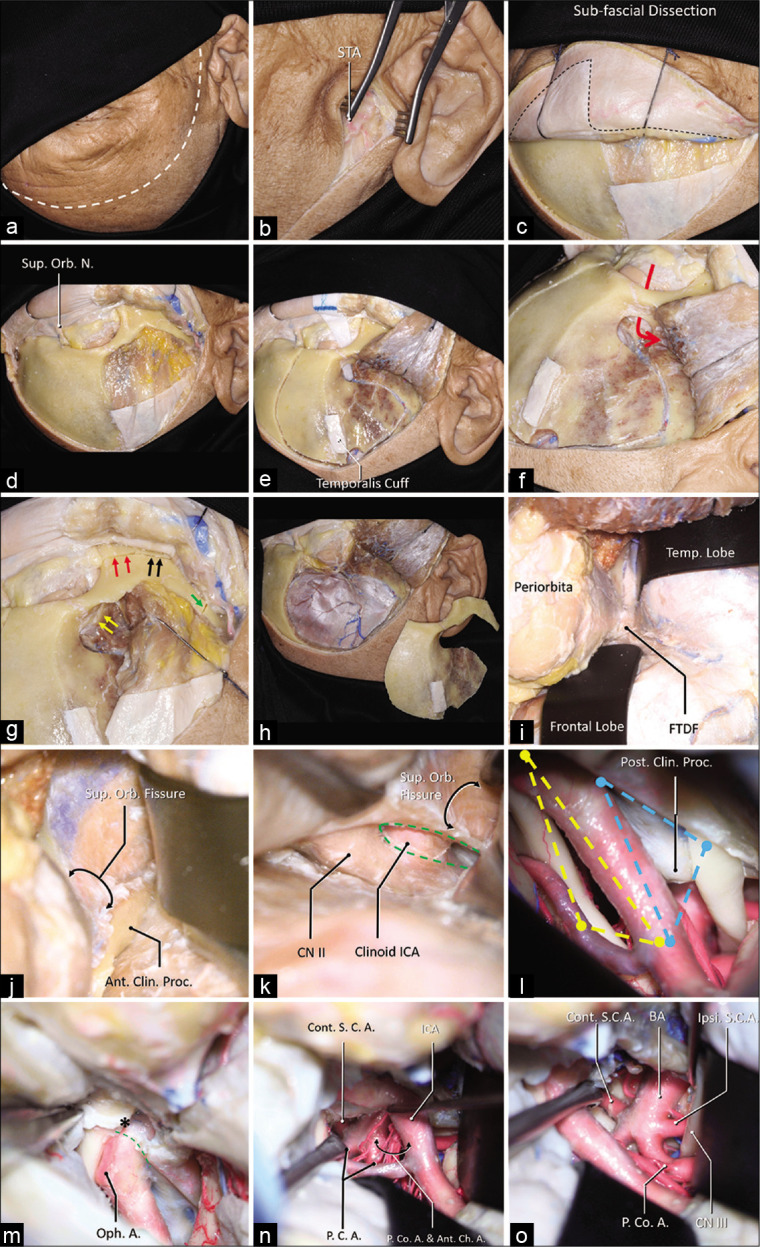
A step-by-step dissection (a) The curvilinear skin incision was demonstrated using the dashed white line, which started from 1 cm in front of the tragus and ended at the contralateral mid-pupil line. (b) The superficial temporal artery (STA) was found in the subcutaneous layer. (c) A sub-fascial dissection was performed to keep the continuation of the periosteum superior to the temporal line and the superior temporoparietal fascia inferior to the temporal line (dashed line). (d) The orbit and the zygoma were demonstrated, and the superior orbital nerve was dissected free. (e) A temporalis muscle cuff was left on the skull for reapproximation, a MacCarty keyhole at the level of the frontozygomatic suture was placed, which connects both anterior skull base and the orbit (f), the first cut was placed. (g) The MacCarty keyhole was connected to the inferior orbit fissure (yellow arrows), there were three separated cuts on the zygoma: 1, the anterior half of the zygoma was cut connecting the inferior orbital fissure (red arrows); 2, the posterior half of the zygoma was cut connecting the infratemporal fossa (black arrows); 3, the zygomatic arch was disconnected (green arrow). (h) A general view of the one-piece orbitozygomatic craniotomy. (i) After the pretemporal extradural exploration, the frontotemporal dural fold (FTDF) was demonstrated. (j) the superior orbital fissure and the nerves within were shown to be inferior to the anterior clinoid process. (k) After the anterior clinoidectomy (green dashed line), the clinoid segment of the carotid artery was demonstrated. (l) The intradural exposure showed the opitco-carotid triangle (yellow dashed line) and the carotico-oculomotor triangle (blue dashed line). (m) The distal dural ring (green dashed line) was dissected, the apex of the OCT (asterisk) was demonstrated. After the posterior clinoidectomy, the exposure to the basilar system via the OCT (n) and the COT (o) was demonstrated. Abbreviations: A., artery; Ant., anterior; Ant. Ch. A., anterior choroidal artery; BA, basilar artery; Clin., clinoid; CN, cranial nerve; Cont., contralateral; FTDF, frontotemporal dural fold; ICA, internal carotid artery; Ipsi., ipsilateral; Orb., orbital; N., nerve; Oph., ophthalmic; P.C.A., posterior cerebral artery; P. Co. A., posterior communicating artery; proc., process; S.C.A., superior cerebellar artery; STA, superficial temporal artery; Sup., superior; temp., temporal.
Quantitative measurements
In a transsylvian approach, the AIW was measured as the distance between the JAPPL on both sides.[1]
The OCT was measured using the following 3 points as three vertices[7] [Figure 1l]: The junction of the optic tract and A1, the ICA bifurcation, and the junction of the ICA and optic nerve [can be proximal to the distal dural ring after drilling the optic strut, Figure 1m].
The COT was measured using the following 3 points as three vertices[16] [Figure 1l]: The dural entry of the oculomotor nerve, ICA bifurcation, and lateral edge of the distal dural ring.
A modified superior transcavernous approach was subsequently performed by opening the oculomotor triangle, and a posterior clinoidectomy was performed. The maximal width of the OCT was measured after mobilizing the ICA laterally [Figure 1n] and the maximal width of the COT was measured after mobilizing the ICA medially and the oculomotor nerve laterally [Figure 1o]. The AIW can be measured between JAPPL [Figure2].
Statistical analysis
In a study which investigated 100 specimens,[1] the AIW (was referred to as the “anterior notch width” in this study) had a mean distance of 26.6 ± 2.7 mm, a median of 26.7 mm, a 25 percentile of 24.5 mm, and a 75 percentile of 28.0 mm. Therefore, we selected 26.6 mm as a cutoff to group the specimens into wide (with an AIW larger than 26.6 mm) and narrow groups (with an AIW smaller than 26.6 mm). The area of the OCT and COT was compared between the narrow (Group A) and wide (Group B) groups. Consequently, the maximal widths of the OCT and COT were compared between the two groups.
As the area and the width may not have standard normal distribution, a Mann–Whitney U-test was used in the comparison. The results were presented as “mean ± standard deviation.” P < 0.05 was considered as statistically significant. The statistical analysis was performed using PASW Statistics 18.0.0 (IBM Corporation, Armonk, New York).
The Institutional Review Board approval was neither required for the anatomical cadaver dissection study and nor for the case examples description due to retrospective review and lack of identification on imaging studies.
RESULTS
The AIW was 24.3 ± 1.23 mm in the narrow group and 29.3 ± 1.59 in the wide group. No difference between the two groups was reported in terms of the area of the OCT (50.9 ± 19.22 mm vs. 63.5 ± 15.53 mm, P = 0.20), the maximal width of the OCT (6.6 ± 1.89 vs. 6.5 ± 1.38 mm, P = 1.00), or the maximal width of the COT (11.7 ± 2.06 vs. 12.2 ± 2.32 mm, P = 0.50).
The COT in the wide group was shown to have a significantly larger area compared to the COT in the narrow group (38.4 ± 12.64 vs. 58.3 ± 15.72 mm, P < 0.01).
Case examples
The AIW was measured in different clinical cases with lesions at interpeduncular fossa operated by the corresponding author as follows:
Case 1
A 56-year-old right-handed female with high blood pressure difficult to control was evaluated for unruptured 8 mm basilar apex aneurysm with high bifurcation (4 mm above posterior clinoid) [Figure 3]. AIW on MRI and CTA was 26.0 mm [Figure 4]. After an elective follow-up and discussion about the endovascular and surgical clipping options, the patient underwent surgical clipping of the aneurysm with a right- sided two-piece cranio-orbitozygomatic approach, extradural anterior clinoidectomy, intraoperative angiogram, and lumbar drain placement. The aneurysm was well exposed with transsylvian dissection alone [Figure 5]. Postoperatively, the patient developed transient partial right oculomotor palsy that completely resolved 3 weeks after surgery. Since AIW was narrow, surgical access was facilitated due to height of the basilar apex aneurysm [Figure 6].
Figure 3:
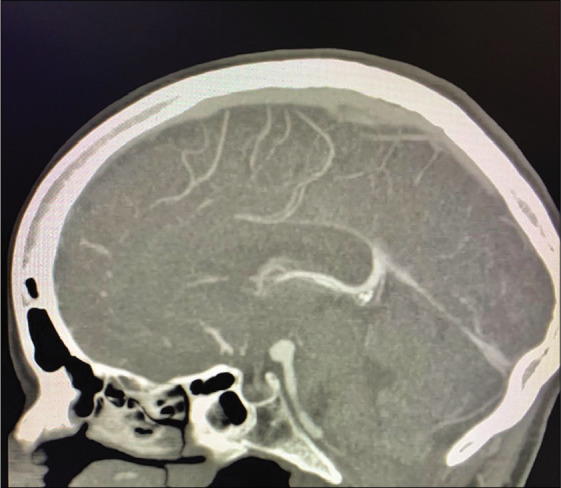
Case 1, sagittal view on CTA with high bifurcation of basilar artery in relation to posterior clinoid. Abbreviations: CTA, computed tomography angiogram.
Figure 4:
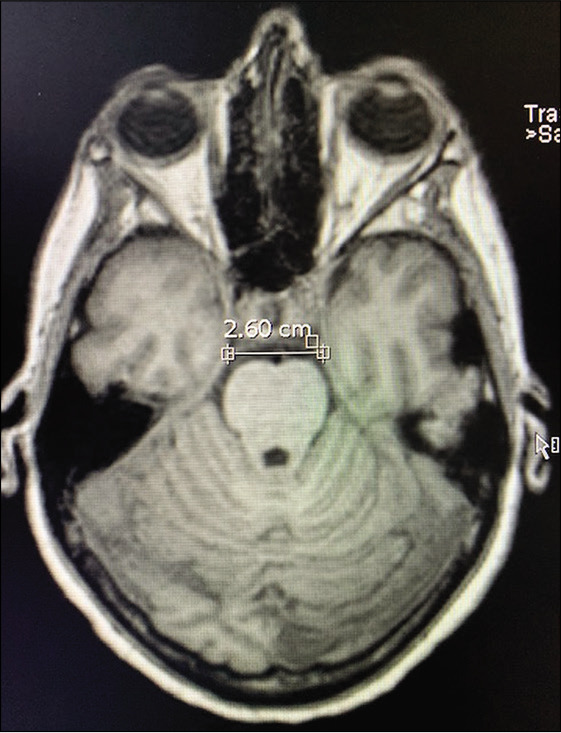
Case 1, AIW measured on MRI. Abbreviations: AIW, anterior incisural width; MRI magnetic resonance imaging.
Figure 5:
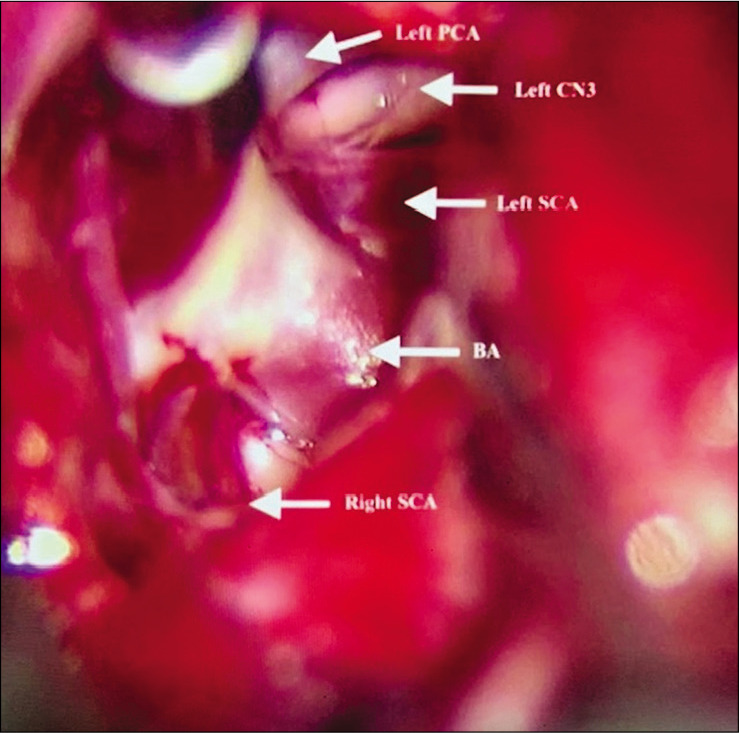
Case 1, microsurgical view at interpeduncular fossa prior to aneurysm clipping. PCA Posterior cerebral artery; CN3 Cranial nerve 3 (oculomotor); SCA Superior cerebellar artery; BA Basilar artery.
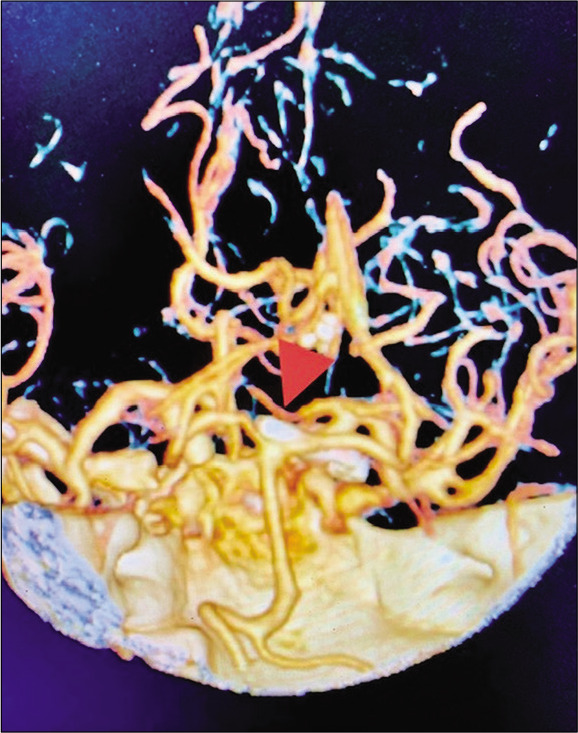
Figure 6:
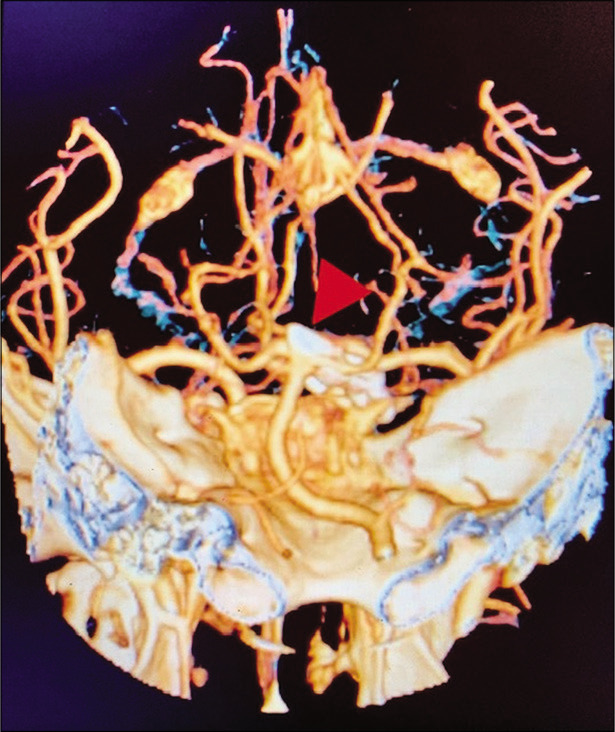
Case 1, three-dimensional CTA post basilar apex aneurysm clipping (arrowhead). Abbreviations: CTA, computed tomography angiogram.
Figure 2:
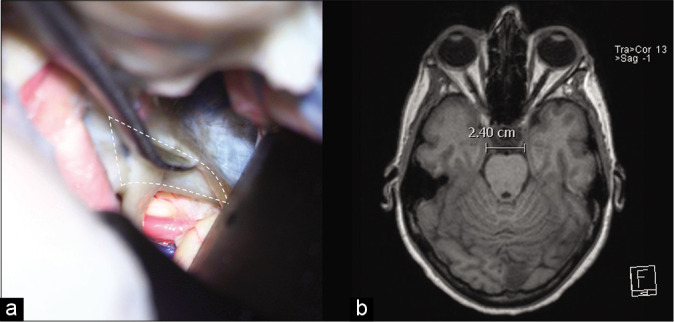
(a) The junction of the anterior petroclinoid ligament and the posterior petroclinoid ligament JAPPL was demonstrated, the anterior incisural width was measured as the distance of the JAPPL on both sides. (b) Illustration of the measurement of the AIW on an axial MRI scan. Abbreviations: JAPPL, junction of anterior and posterior petroclinoid ligaments; AIW, anterior incisural width.
Case 2
A 69-year-old right-handed female with no relevant medical history developed a sudden onset of severe headache and was found with subarachnoid hemorrhage Hunt and Hess 2, Fisher 3 with 3 mm basilar apex aneurysm rupture. The patient had significant tortuosity of vertebral arteries and, additionally, given the relatively small size of the aneurysm, our neuroendovascular team found it difficult to perform endovascular treatment [Figure 7]. The AIW estimate on CTA was 25.0 mm [Figure 8]. The patient underwent external ventricular drain placement, right-sided skull base approach with transzygomatic, orbitocranial, extradural anterior clinoidectomy, pretemporal, and partial transcavernous approach with partial posterior clinoidectomy [Figure 9] for basilar apex aneurysm clipping. The patient developed complete and transient oculomotor palsy that recovered entirely in 6 months. The estimate AIW was narrow and required partial transcavernous exposure [Figure 10].
Figure 7:
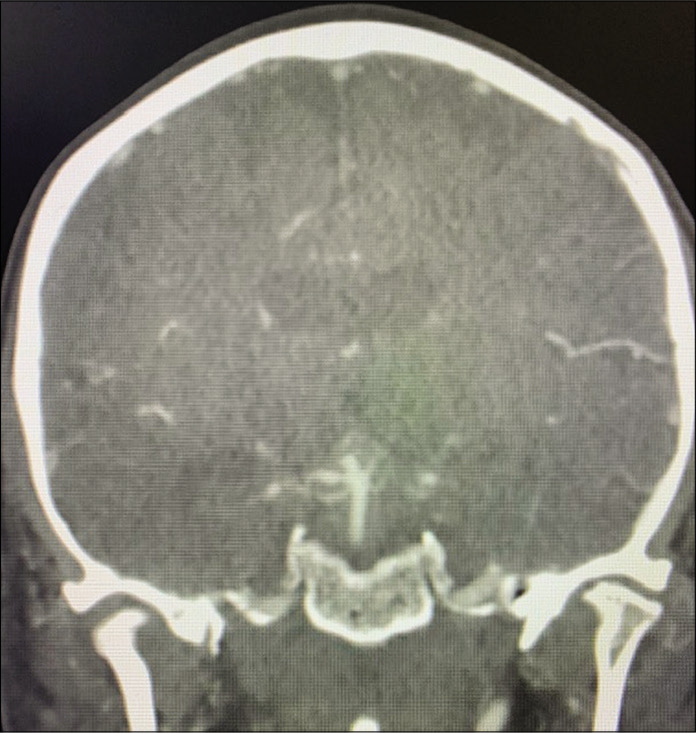
Case 2, coronal view on preoperative CTA. Abbreviations: CTA, computed tomography angiogram.
Figure 8:
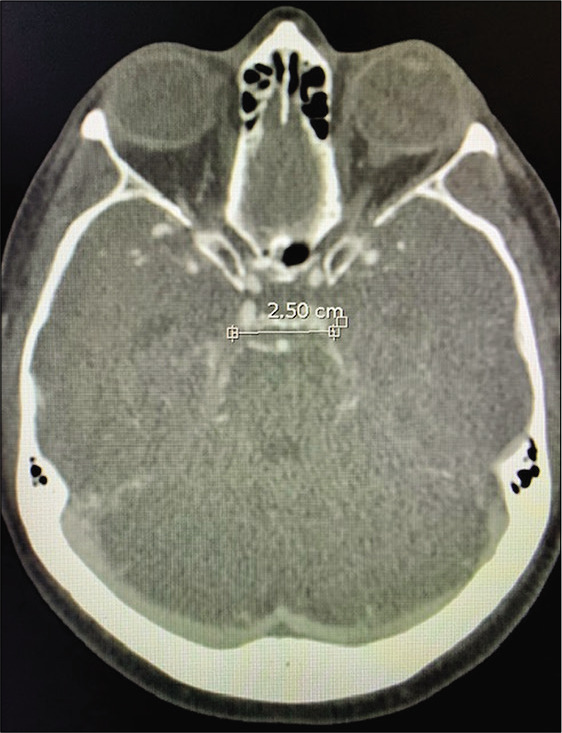
Case 2, AIW measured on CTA. Abbreviations: AIW, anterior incisural width; CTA, computed tomography angiogram.
Figure 9:
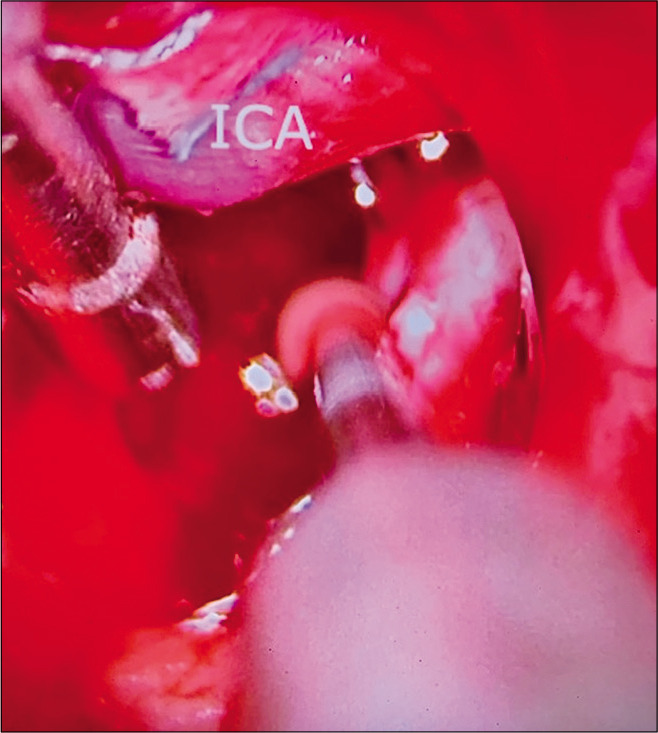
Case 2, microsurgical view at interpeduncular fossa during posterior clinoidectomy.
Figure 10:
Case 2, microsurgical view at interpeduncular fossa during posterior clinoidectomy.
Case 3
A 50-year-old female with visual field deficit – preoperative MRI showed a 2 × 1.3 × 1.3 cm (AP, transverse, and craniocaudal) extra-axial mass extending from the interpeduncular cistern to the left posterior suprasellar space, displacing the left cerebral peduncle, and the ICA laterally, and the left optic nerve and chiasm superiorly [Figure 11]. CTA was negative for vascular lesions. AIW on MRI and CTA was 29.1 mm [Figure 12]. The patient underwent a left-sided skull base approach with orbital cranial approach, pretemporal approach, extradural anterior clinoidectomy, and transsylvian microsurgical partial tumor resection due to capsule adhesions to the ventral aspect of the optic nerve, chiasm, left oculomotor nerve, and basilar artery trunk at interpeduncular fossa. Intraoperatively, the lesion caused an inferior displacement of the left P1 segment of the posterior cerebral artery, and lateral displacement of the supraclinoid ICA and left posterior communicating artery [Figure 13]. The pathology report was consistent with craniopharyngioma. Postoperatively, the patient developed left oculomotor nerve palsy with subtotal recovery at 3 months after surgery. The wide anterior incisura favored surgical exploration and subtotal resection through a transsylvian and pretemporal approach [Figure 14].
Figure 11:
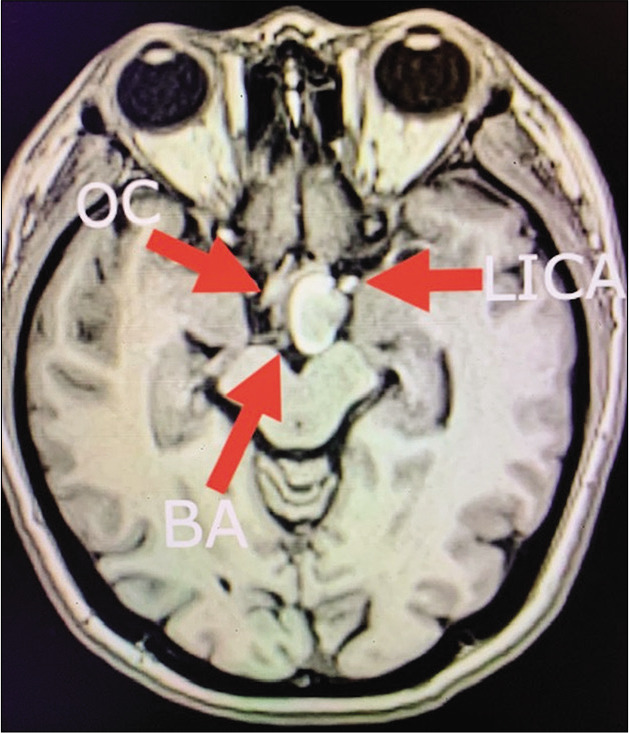
Case 3, preoperative brain MRI without contrast showing heterogenous hyperintense lesion displacing medially the optic chiasm (OC), laterally the left internal carotid artery (LICA), and located ventral and lateral to basilar artery bifurcation (BA). Abbreviations: MRI, magnetic resonance imaging; OC, optic chiasm; LICA, left internal carotid artery; BA, Basilar artery.
Figure 12:
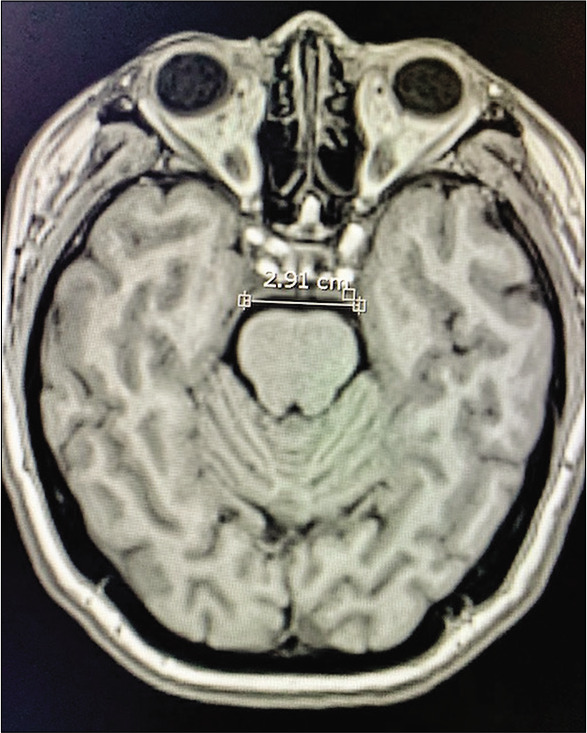
Case 3, AIW measured on MRI. Abbreviations: AIW, anterior incisural width; MRI magnetic resonance imaging.
Figure 13:
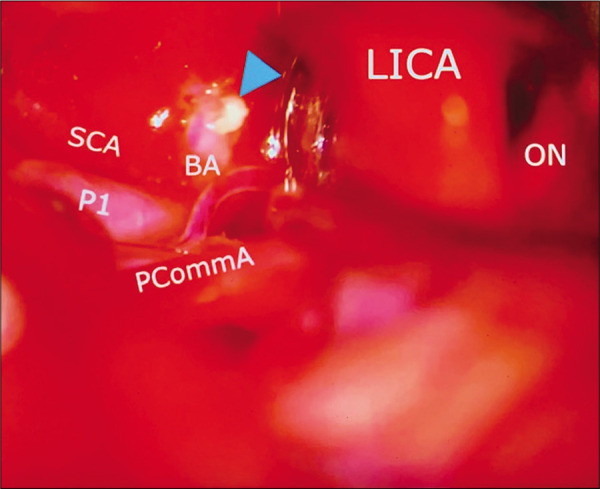
Case 3, left sided microsurgical view at COT displacing the internal carotid artery (LICA) anteriorly, and below posterior communicant artery (PCoA), exposing the basilar artery apex (BA), left posterior cerebral artery at P1 segment (P1), and left superior cerebellar artery (SCA). A small segment of residual tumor is left attached to basilar artery (arrowhead). Abbreviations: COT, carotico-oculomotor triangle; LICA, left internal carotid artery; PCoA, posterior communicant artery; BA, basilar artery apex; P1, posterior cerebral artery at P1 segment; SCA, superior cerebellar artery; ON, optic nerve.
Figure 14:
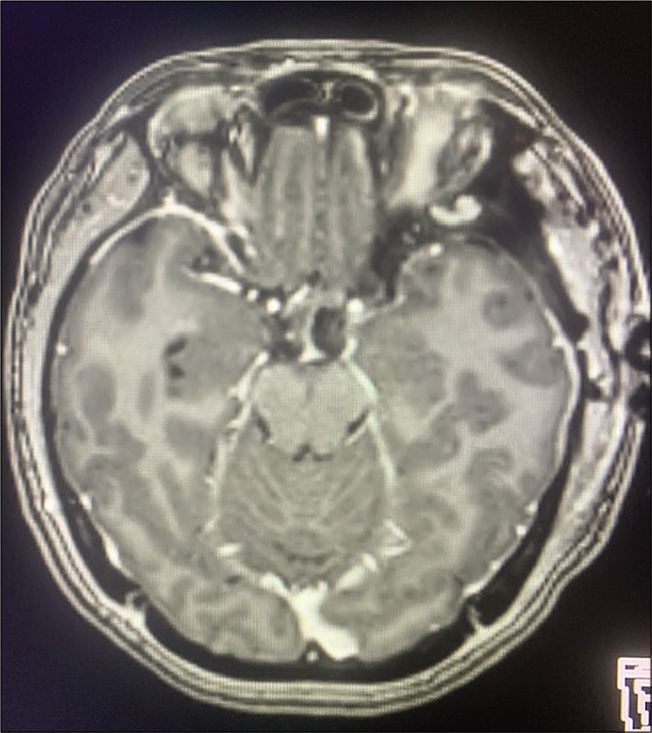
Case 3, postoperative brain MRI with contrast after lesion resection. Abbreviations: MRI magnetic resonance imaging.
DISCUSSION
In this manuscript, we drew the conclusion that the width of the anterior incisural space was correlated with the OCT, which can be measured preoperatively as an indicator for predicting the intradural exposure to the interpeduncular space.
Approaches and the anatomical triangles
Although there are slightly different modifications within them, lateral transcranial approaches accessing the prepontine, crural, and interpeduncular cisterns can be broadly classified as the “transsylvian approach”[4,12] and the “transcavernous approach.”[6,9,14] The key difference is whether the roof (the oculomotor triangle) and upper lateral wall of the cavernous sinus is being dissected. As such, by opening the oculomotor triangle, the oculomotor nerve can be mobilized down and the posterior clinoid process can be removed. If needed, the trochlear nerve can be dissected giving access to supratrochlear and infratrochlear triangles; by performing these two maneuvers, the posterior genu of the cavernous ICA becomes the only structure preventing access to the basilar artery proximally in low lying basilar artery bifurcation.[14,17] The transcavernous approach offers significantly larger exposure than the transsylvian approach.[9] However, this approach is technically more demanding with an inherent higher rate of transient oculomotor nerve palsy.[3,14] Moreover, pretemporal extradural exploration and entering the cavernous sinus require deep microsurgical anatomy knowledge and high microsurgical training; otherwise, it could jeopardize other cranial nerves and eloquent neurovascular structures in the cavernous sinus.
Regardless of the approach, two intradural anatomical triangles constitute major anatomical spaces through which the target region can be accessed.
Opening the OCT utilizes the space between the optic nerve and the ICA; by performing the anterior clinoidectomy and dissecting the distal and proximal dural rings, the vertex of the OCT can be extended proximally along the ICA to increase the area of the OCT.[7]
The COT[16] utilizes the space between the ICA and the oculomotor nerve. In a transsylvian approach, the posterior clinoid process may be prominent and hence obstruct the exposure to the basilar artery proximally [Figure 1l]. Furthermore, a posterior clinoidectomy can increase the proximal exposure on the basilar artery.[9] By dissecting the distal and proximal dural rings, the clinoid segment of the ICA connects with the intradural ICA, which offers more freedom to mobilize the ICA medially. The dura entry of the oculomotor nerve is spatially fixed at the oculomotor triangle, consisting of the anterior petroclinoid ligament, the posterior petroclinoid ligament, and the interclinoid ligament.[13] In a transcavernous approach, by opening the oculomotor triangle and dissecting the oculomotor nerve from the arachnoid adhesion at the oculomotor cistern,[8,17] the oculomotor nerve can be safely mobilized laterally, which offers more proximal exposure on the basilar artery through the COT [Figure 1o].[9]
Risk to take in approach selection
A transcavernous approach is optimal in terms of the exposure on the basilar artery; however, it requires pretemporal extradural exploration [Figure 1i] and is skill demanding. Some high-riding basilar aneurysms may not require a full transcavernous approach to obtain the proximal control, dome dissection, and eventually, clip reconstruction; however, the anatomical configuration of the triangle is highly variable, and the exposure offered through the triangles in a transsylvian approach is majorly dependent on the variable configuration.[11] However, no indicator can predict the configuration of the triangles preoperatively.
The AIW as a preoperative indicator
The distance between the JAPPL on both sides represents the width of the anterior incisural space. In our study, we reported that the AIW was correlated with the size of the COT (in the transsylvian approach), but not related with the maximum widths of the OCT and COT (in the transcavernous approach), which meant that in a transcavernous approach with the OCT and COT maximally opened, the narrow group offered similar exposure compared to the wide group. The narrow group had a smaller COT area, which is an unfavorable factor. Therefore, a transcavernous approach may be required, as in the transcavernous approach, where two groups offer similarly considerable exposure.
In preoperative settings, measuring the AIW can offer additional information to predict the size of the COT and be helpful in determining and adequately preparing if a transcavernous approach should be selected, although it is important to consider that this analysis of our case examples was retrospective.
For example, the patient on Case 1 with a high bifurcation basilar apex aneurysm had a narrow AIW of 26.0 mm, but surgical access was facilitated due to height of the basilar apex bifurcation regardless of AIW measurement; however, other factors were relevant, such as length of PCommA, no SAH, and separation between the posterior clinoid and basilar trunk. In Case 2, the estimate AIW was narrow (25.0 mm) and given basilar apex bifurcation at the level of posterior clinoid, required partial transcavernous exposure for adequate basilar apex aneurysm clipping regardless of AIW measurement. In Case 3, the wide anterior incisura of 29.1 mm, which in retrospective, we consider was a favorable factor, reflected by a wide microsurgical view for subtotal resection of tumor through the transsylvian and pretemporal approach.
Limitations of the study
The present study was performed using cadaveric specimens, the texture of which differs from that of a live tissue, in which setting the exposure can be slightly different than in real microsurgical interventions. We recognize that a limitation in our cadaveric study is the number of specimens; a larger number could have given modification of our results. More factors are involved in the approach selection, such as the height of the basilar artery bifurcation, the prominence of the posterior clinoid process, the diameter and length of the posterior communicating artery, and distance between dorsal clivus and posterior clinoid to ventral basilar artery trunk. Therefore, the approach selection should be carried out in a highly individualized fashion, with comprehensive consideration of all possible factors. Finally, a brain MRI is more favorable than CTA to clearly identify AIW. However, in practical scenarios, it is uncommon for patients with aneurysm lesions to undergo an MRI scan, which is also financially disadvantageous.
CONCLUSION
The AIW is a potential preoperative indicator of the size of the COT. An AIW <26.6 mm is considered “narrow,” which is related to a small area of the COT. We consider this an unfavorable factor for a transsylvian approach in lesions located at interpeduncular cistern, and a transcavernous approach can be considered under this circumstance. The AIW is only one element that we suggest can be taken into account during preoperative planning among multiple other elements for surgery in these areas. A larger series with measurement of AIW’s can help to define its real use as a predictive tool for favorable microsurgical view and approach selection.
Footnotes
How to cite this article: Zhao X, Labib M, Ramanathan D, Eastin TM, Song M, Little AS, et al. The anterior incisural width as a preoperative indicator for intradural space evaluation: An anatomical investigation. Surg Neurol Int 2020;11:207.
Contributor Information
Xiaochun Zhao, Email: kyle.g0704@gmail.com.
Mohamed Labib, Email: mohamed.labib@gmail.com.
Dinesh Ramanathan, Email: dramanathan@llu.edu.
Timothy Marc Eastin, Email: meastin@llu.edu.
Minwoo Song, Email: minsong@llu.edu.
Andrew S. Little, Email: andrew.little@barrowbrainandspine.com.
Mark C. Preul, Email: mark.preul@barrowneuro.org.
Michael T. Lawton, Email: michael.lawton@barrowbrainandspine.com.
Miguel Angel Lopez-Gonzalez, Email: mlopezgonzalez@llu.edu.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Adler DE, Milhorat TH. The tentorial notch: Anatomical variation, morphometric analysis, and classification in 100 human autopsy cases. J Neurosurg. 2002;96:1103–12. doi: 10.3171/jns.2002.96.6.1103. [DOI] [PubMed] [Google Scholar]
- 2.Aziz KA, Froelich SC, Cohen PL, Sanan A, Keller JT, van Loveren HR. The one-piece orbitozygomatic approach: The MacCarty burr hole and the inferior orbital fissure as keys to technique and application. Acta Neurochir (Wien) 2002;144:15–24. doi: 10.1007/s701-002-8270-1. [DOI] [PubMed] [Google Scholar]
- 3.Basma J, Ryttlefors M, Latini F, Pravdenkova S, Krisht A. Mobilization of the transcavernous oculomotor nerve during basilar aneurysm surgery: Biomechanical bases for better outcome. Neurosurgery. 2014;10(Suppl 1):106–15. doi: 10.1227/NEU.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 4.Bendok BR, Getch CC, Parkinson R, O’Shaughnessy BA, Batjer HH. Extended lateral transsylvian approach for basilar bifurcation aneurysms. Neurosurgery. 2004;55:174–8. doi: 10.1227/01.neu.0000126948.21288.af. [DOI] [PubMed] [Google Scholar]
- 5.Coscarella E, Başkaya MK, Morcos JJ. An alternative extradural exposure to the anterior clinoid process: The superior orbital fissure as a surgical corridor. Neurosurgery. 2003;53:162–7. doi: 10.1227/01.neu.0000068866.22176.07. [DOI] [PubMed] [Google Scholar]
- 6.Dolenc VV, Škrap M, Šušteršič J, Skrbec M, Morina A. A transcavernous-transsellar approach to the basilar tip aneurysms. Br J Neurosurg. 1987;1:251–9. doi: 10.3109/02688698709035309. [DOI] [PubMed] [Google Scholar]
- 7.Evans JJ, Hwang YS, Lee JH. Pre-versus post-anterior clinoidectomy measurements of the optic nerve, internal carotid artery, and opticocarotid triangle: A cadaveric morphometric study. Neurosurgery. 2000;46:1018–23. [PubMed] [Google Scholar]
- 8.Everton KL, Rassner UA, Osborn AG, Harnsberger HR. The oculomotor cistern: Anatomy and high-resolution imaging. AJNR Am J Neuroradiol. 2008;29:1344–8. doi: 10.3174/ajnr.A1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figueiredo EG, Zabramski JM, Deshmukh P, Crawford NR, Preul MC, Spetzler RF. Anatomical and quantitative description of the transcavernous approach to interpeduncular and prepontine cisterns. J Neurosurg. 2006;104:957–64. doi: 10.3171/jns.2006.104.6.957. [DOI] [PubMed] [Google Scholar]
- 10.Froelich SC, Aziz KM, Levine NB, Theodosopoulos PV, van Loveren HR, Keller JT. Refinement of the extradural anterior clinoidectomy: Surgical anatomy of the orbitotemporal periosteal fold. Neurosurgery. 2007;61(Suppl 2):179–85. doi: 10.1227/01.neu.0000303215.76477.cd. [DOI] [PubMed] [Google Scholar]
- 11.Gibo H, Lenkey C, Rhoton AL. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981;55:560–74. doi: 10.3171/jns.1981.55.4.0560. [DOI] [PubMed] [Google Scholar]
- 12.Hsu FP, Clatterbuck RE, Spetzler RF. Orbitozygomatic approach to basilar apex aneurysms. Neurosurgery. 2005;56(Suppl 1):172–7. doi: 10.1227/01.neu.0000144317.44745.d1. [DOI] [PubMed] [Google Scholar]
- 13.Isolan GR, Krayenbühl N, de Oliveira E, Al-Mefty O. Microsurgical anatomy of the cavernous sinus: Measurements of the triangles in and around it. Skull Base. 2007;17:357–67. doi: 10.1055/s-2007-985194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krisht AF, Kadri PA. Surgical clipping of complex basilar apex aneurysms: A strategy for successful outcome using the pretemporal transzygomatic transcavernous approach. Neurosurgery. 2005;56(Suppl 2):261–73. doi: 10.1227/01.neu.0000156785.63530.4e. [DOI] [PubMed] [Google Scholar]
- 15.Lemole GM, Henn JS, Zabramski JM, Spetzler RF. Modifications to the orbitozygomatic approach. Technical note. J Neurosurg. 2003;99:924–30. doi: 10.3171/jns.2003.99.5.0924. [DOI] [PubMed] [Google Scholar]
- 16.Sade B, Kweon CY, Evans JJ, Lee JH. Enhanced exposure of carotico-oculomotor triangle following extradural anterior clinoidectomy: A comparative anatomical study. Skull Base. 2005;15:157–61. doi: 10.1055/s-2005-871523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tayebi MA, Borba LM, Zhao X, Lawton MT, Preul MC. Transcavernous approach to the upper basilar and retroclival area-cadaveric surgical simulation video: 2-Dimensional operative video. Oper Neurosurg (Hagerstown) 2019;17:E251. doi: 10.1093/ons/opz031. [DOI] [PubMed] [Google Scholar]
- 18.Zabramski JM, Kiriş T, Sankhla SK, Cabiol J, Spetzler RF. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89:336–41. doi: 10.3171/jns.1998.89.2.0336. [DOI] [PubMed] [Google Scholar]


