Abstract
A Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) has become a pandemic disease named Coronavirus Disease-19 (COVID-19) of epochal dimension. The clinical spectrum of COVID-19 is wide, ranging from asymptomatic forms to severe pneumonia, sepsis and multiple organ dysfunction syndromes resulting in poor outcomes.
Among the various consequences of severe COVID-19, cardiovascular (CV) collapse appears the most serious and potentially lethal. On the other hand, pre-existent CV comorbidities are also associated with higher mortality. The most reliable hypothetical pathogenetic mechanism for CV complications and cardiac injury in severe COVID-19 patients appears to be a sustained endothelial dysfunction, caused by the interplay of inflammation and coagulation.
In this review, we survey papers addressing issues related to severe COVID-19, characterized by enhanced lung microvascular loss, hypercytokinemia, hypoxemia and thrombosis. We discuss about how the virus-induced downregulation of the angiotensin converting enzyme-2 (ACE2) receptor, used to enter the host cell, could affect the renin-angiotensin system, attempting to clarify the doubts about the use of ACE inhibitors and Angiotensin-II receptor blockers in COVID-19 patients. Finally, we point out how the delicate and physiological homeostatic function of the endothelium, which turns into a disastrous battlefield of the complex interaction between “cytokine and coagulative storms”, can be irreparably compromised and result in systemic inflammatory complications.
Keywords: COVID-19, Endothelial dysfunction, Thrombosis, Inflammation, Cardiac injury
1. Introduction
Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) is the pathogen agent of a pandemic disease named coronavirus disease 2019 (COVID-19) that has become a global and epochal challenge [1]. SARS-CoV-2 is a single-stranded RNA virus with surface binding glycoproteins called “spikes”. It shares a highly similar gene sequence with SARS-CoV, virus that caused an epidemic in 2003, with some similarities in the epidemiology of infection and disease clinical features [2,3]. An initial overview from China including 72,314 patients revealed several important epidemiological and clinical features of COVID-19 [4]. The clinical spectrum of COVID-19 is very wide, and ranges from the absence of overt symptoms or only mild signs of upper respiratory tract infection in 81% of patients, to severe pneumonia in 14% of the patients or critical conditions characterized by severe acute respiratory distress syndrome (ARDS), systemic infection, septic shock, and multiple organ dysfunction syndrome in 5% of the patients [4]. Since the beginning of the pandemic, around 13 million SARS-CoV-2 positive cases have been detected, and about 600 thousand deaths due to COVID-19 have been recorded, with approximately 4.4% of case fatality rate (confirmed positive deaths/confirmed positive cases) [5]. Population-based mortality estimates vary widely among countries and continue to change as the pandemic continues, probably because of differences in testing strategies, demographics, ethnicity, access to healthcare, timing of peak infection, socioeconomic status, improved disease treatments and study methodology [6,7]. Instead, using the infection fatality rate an index that includes confirmed, suspected, and asymptomatic patients, an increasing number of studies from different regions have estimated global infection fatality rates in the range of 0.5–1% [8].
The fatality rate from COVID-19 is higher among older people, probably reflecting the presence of other diseases, a weaker immune system or simply worse overall health that allows a faster progression of viral infection [4,[8], [9], [10]].
Among various consequences of severe COVID-19, cardiovascular (CV) complications appeared the most serious and potentially lethal [1,11,12].
Acute respiratory infections, including influenza, are well known to be associated with elevated risk of CV diseases (CVD) [13]. This relationship is reciprocal because if, on one hand, viral respiratory infectious diseases, including the previous SARS-CoV outbreak [14], can increase the risk of CV events [15], on the other, the underlying CV comorbidities increase the risk of mortality among patients with infection [16].
A meta-analysis of 46,248 cases revealed that hypertension and diabetes mellitus and global CVD are the most prevalent comorbidities associated with the severity of COVID-19, while ARDS and acute cardiac injury may represent the main complications for the recovery of patients [17].
In a large retrospective study performed on 416 hospitalized patients, cardiac injury was significantly and independently associated with mortality (hazard ratio: 4.26) [18].
Another preprint meta-analysis indicated that acute cardiac injury, hypertension, heart failure, and global CVD were significantly associated with mortality in COVID-19 patients [19].
SARS-CoV-2 enters human cells through the binding of the spike protein with angiotensin converting enzyme-2 (ACE2) [3], a membrane receptor highly expressed in immune or non-immune cells [20,21], and in many organs, including lungs, heart and vessel walls [2]. ACE2 is an important regulator of blood pressure and it is likely that elderly patients with hypertension could have dysregulated ACE2 expression/function, predisposing them to severe conditions and mortality.
Acute lung injury leads to increased cardiac workload, therefore patients with pre-existing CV comorbidities may be at a higher risk of COVID-19 severity [1,22].
However, recent evidence suggests that a more likely pathogenetic hypothesis unifying pre-existing comorbidities and the occurrence of CV events in severe COVID-19 is the endothelial dysfunction due to a direct viral attack but above all to the complex interaction between “cytokines and coagulation storms” that, inside the vessels, can irreparably compromise the integrity and the physiological anti-thrombotic and anti-inflammatory properties of the endothelium [23].
In this review, we summarize current and ongoing evidence supporting endothelial damage underlying the pathophysiology of COVID-19 severity and CV complications, and we focus on one key question: is the endothelial dysfunction the hardest challenge? In an attempt to answer this question, we retrace the steps through which the initially alveolar injury can become systemic through an intensified and dangerous involvement of the vascular endothelium in the interplay between inflammatory and thrombotic events.
2. Virus infection and COVID-19 pathogenesis: what the biopsy results show
Postmortem examination has been crucial in determining the cause of death and in understanding the pathogenesis of COVID-19. The first series of autopsies on patients who died of COVID-19 revealed that the leading process in all cases was consistent with diffuse alveolar damage and airway inflammation [[24], [25], [26]]. Both in patients at an early stage and in a more advanced stage of disease, the pulmonary autopsy revealed formation of hyaline membranes, as well as fibrin deposition, neutrophils, monocytes/macrophages infiltrating air spaces and an extensive thickening of the alveolar wall [24,25,27]. Wang, et al. showed increased accumulation of cytokines and SARS-CoV-2-infected macrophages in the lungs [28]. Type II alveolar cells were found in the alveolar spaces with evident signs of viral cytopathic effects [25]. CD4+ T cells have been reported in aggregates around small vessels, containing platelets and small thrombi with entrapment of numerous neutrophils [25]. From the results of the initial autopsies, it was soon clear that COVID-19 was a systemic pathology affecting the vessels of different anatomical districts and not only the lung. Indeed, the infection caused cell degeneration, necrosis, atrophy, focal haemorrhage and inflammatory cell infiltration in several organs such as heart, vessels, liver, kidneys, spleen, brain and even skin [18,25,26,29]. This evidence is supported by the fact that the ACE2, the gateway of SARS-CoV-2 into endothelial cells, is ubiquitous and not only present in alveolar endothelial cells.
In nine COVID-19 patients out of 26 patients expired from multiple organ dysfunction associated with respiratory failure, the electron microscopic examination showed clusters of viral particles in renal podocytes and epithelium, positive to the viral nucleoprotein-antibody immunostaining [18]. Viral particles have been found in endomyocardial biopsy of a patient with ARDS and cardiogenic shock [29]. Myocardial localization of SARS-CoV-2 implies either a viraemic phase or, alternatively, migration of infected alveolar macrophages from lung to heart [29]. In another series of autopsies, electron microscopy unveiled viral inclusion particles into endothelial cells while histology showed an accumulation of inflammatory cells near the endothelium [30]. SARS-CoV-2 particles were isolated not only from oropharyngeal swab, but also in urine, faecal and serum samples, confirming a systemic viremia [[31], [32], [33], [34]]. This wide spread would explain how the transmission of the virus by respiratory and extra-respiratory ways, including the vascular system, may rapidly worsen the clinical picture of COVID-19.
Recently, several autopsy studies confirmed the results of ante mortem imaging findings, highlighting that small pulmonary vascular changes are a common feature of severe COVID-19 pneumonia. In a study of 11 cases, Lax et al. [35] showed that arterial thrombosis and inflammation of the small segmental or subsegmental vessels on pulmonary autopsies correlated with ante mortem imaging of pulmonary vessel enlargement or thickening on chest computed tomography.
In another study, the lungs from patients with Covid-19 showed distinctive small pulmonary vascular features on histopathology, consisting of severe endothelial injury associated with the presence of intracellular viral particles and deranged cell membranes, widespread vascular thrombosis with microangiopathy and new vessel growth [36]. Therefore, in light of the close correlations between histopathological and radiological findings, combined with the clinical and bio-humoral evidence that will be discussed in the following sections, we can reasonably assume that for at least a significant subset of COVID-19 patients, the underlying cause of respiratory failure and organ dysfunction is essentially thrombosis, inflammation and endothelial dysfunction.
3. Cell entry to SARS-CoV-2 invasion: the ACE2 receptor
3.1. ACE2 receptor and renin angiotensin system
ACE2 has been identified as the main receptor used by SARS-CoV and SARS-CoV-2 to enter human cells [3]. ACE2 is an integral transmembrane protein, anchored at the apical surface of the cell, with a catalytic domain located at the extracellular side of the cell, which can be cleaved and released into bloodstream by ADAM17 (a disintegrin and metalloproteinase domain-containing protein 17). The spike protein, a viral surface glyprotein, assembles as trimer and plays the most important role in viral attachment, fusion and entry [3]. It is composed of a short intracellular tail, a transmembrane anchor, and a large ectodomain that consists of a receptor binding S1 subunit and a membrane-fusing S2 subunit. The S1 subunit contains a receptor binding domain, that binds the cell surface ACE2 receptor for entry into the host cells [3,37]. After receptor binding, spike protein is cleaved by proteases, such as transmembrane protein serine 2 (TMPRSS2) and furin, crucial for membrane fusion with the host cell and infection [37]. The viral genome RNA replicates in the host cell cytoplasm leading to newly formed genomic RNA, which is processed into virion-containing vesicles that fuse with the cell membrane to release the virus.
ACE2 is ubiquitous and widely expressed in lung, heart, vascular system (endothelial cells and smooth muscle cells), gut, kidney, testis and brain, providing a mechanism for the multiple-organ dysfunction as observed in COVID-19 patients [38,39].
In the respiratory tract, elevated ACE2 expression has been identified in type II alveolar cells (representing about 80% of all ACE2-expressing cells) [20], in nasal and oral mucosa as well as in alveolar macrophages [21]. In the lungs, local activation of RAS can influence the pathogenesis of lung damage through multiple mechanisms, such as an increase in vascular permeability and changes in alveolar epithelial cells [40].
ACE2 is also highly expressed in pericytes, undifferentiated and contractile cells that partially surround the capillary endothelial cells and support the capillary integrity, and this could induce increased microvascular damage [41]. Moreover, ACE2 is overexpressed in cardiac myocytes from failing human hearts, suggesting a plausible explanation for a higher infectivity of virus and a higher mortality in patients with heart failure [20].
ACE2 is indeed a key component of the renin angiotensin system (RAS), a key hormonal system involved in the CV pathogenesis. The RAS system is characterized by proteolytic cascade reactions that ultimately convert Angiotensin-I (Ang-I) to vasoconstricting and proinflammatory Ang-II by ACE enzyme. Ang-II binds to specific receptors – Ang-type-1 (AT1) and Ang-type-2 (AT2) – on the cell membrane exerting opposite effects on respiratory and CV system [42]. In the RAS system, ACE2 acts as a carboxypeptidase able to counter-regulate the action of ACE, modulating the balance between vasoconstrictors and vasodilators [43]. ACE2 cleaves a single residue from Ang I to generate Ang 1–9, and degrades Ang-II, the main effector of the RAS, to the vasodilator Ang (1–7). Ang (1–7) binds to G-protein coupled Mas receptor, promoting vasodilation, anti-inflammatory and antifibrotic effects [42].
3.2. Interactions of SARS-CoV-2 with ACE2: the debate about the use of RAS inhibitors is dissolving
ACE2 counteracts the effects of Ang-II in states with excessive activation of RAS such as hypertension, congestive heart failure, atherosclerosis, metabolic disorders and older age [42]. These conditions are characterized by an upregulation of ACE/Ang II/AT1R axis and a downregulation of ACE2/Ang-(1–7)/Mas axis, commonly balanced by drugs such as ACE inhibitors (ACEIs) and AT-1 receptor blockers (ARBs) [44].
Since fatality rate is high in COVID-19 patients with underlying comorbidities such as hypertension, cardiometabolic disorders, heart failure and elderly, a heated debate has sparked in the scientific community about the appropriateness of their use in the presence of infection.
Concerns and doubts about their use on COVID-19 patients arise from animal studies suggesting that ARBs and ACEIs could increase the expression of ACE2 in the CV and renal systems [[45], [46], [47]]. Hence the doubt that an increase in ACE2 expression could aggravate the SARS-CoV-2 infection, due to a greater probability of entering the host cell and, consequently, the severity of COVID-19 in hypertensive and CVD patients who take these medications [48,49]. However, we have to emphasize that it is not yet known and even in animal models, whether overexpression of ACE2 actually facilitates greater engagement and entry of SARS-CoV, SARS-CoV-2 or other viruses.
Current evidences do not support the idea that treatment with ACEIs or ARBS increases ACE2 protein expression and SARS-CoV-2 virus infectivity and/or severity of COVID-19 in humans [50]. Recent results have shown that ACEI use was associated with decreased ACE2 and TMPRSS2 expression in human lungs but not with ADAM17 expression, while neither cardiometabolic diseases (eg, hypertension, diabetes, and cardiac diseases) nor ARBs were associated with altered expression of these genes [51]. These results suggest that an increased risk of COVID-19 in patients with cardiometabolic comorbidity is not related to upregulation of the SARS-CoV-2 receptor or proteases in the lung.
In theory, ACE2 downregulation might reduce the risk of SARS-CoV-2 infection because of reduced virus receptor availability. However, experimental animal studies suggested that the infection by SARS-CoV, which shares the same ACE2 receptor for cell entry, reduced lung ACE2 expression, amplifying acute lung injury because of an imbalance in Ang- II or AT1 signalling [52,53]. Accordantly, an accumulation of non-competitive Ang-II occurs, with consequent severe adverse effects including a rapid vasoconstriction and limited pulmonary circulation, leading to increased inflammatory responses, vascular permeability and pulmonary oedema. Thus, in elderly patients with cardiometabolic comorbidities in whom ACE2/Ang-(1–7)/Mas axis is already strained, the further dramatic decrease due to viral consumption of ACE2 used to enter could really induce pulmonary inflammation and thrombosis as unwanted effects of enhanced and unopposed Ang II effects via the ACE/Ang-II/AT1 receptor axis.
Some clinical studies have shown that ARBs have potential benefits in the prevention and treatment of lung injury caused by COVID-19 [54,55]. In contrast, there is no clinical or experimental evidence supporting that ACEIs and ARBs either augment the susceptibility to SARS-CoV-2 or aggravate the severity and outcomes of COVID-19, currently. A Retrospective cohort study showed no association between ACEI or ARB use and COVID-19 test positivity supporting current professional society guidelines not to discontinue ACEIs or ARBs in the setting of the COVID-19 pandemic [56].
A recent meta-analysis performed on limited retrospective studies in patients with COVID-19 investigating the effect of ACEI/ARBs on disease severity and death as outcomes, suggests that use of these medications may reduce the odds of mortality [57].
4. SARS-CoV-2 attacks the lining of blood vessels
The endothelium plays an important role in the blood vessel physiology regulating the vascular tone and preserving the vascular homoeostasis through autocrine, paracrine, and hormone-like mechanisms [58]. Endothelial dysfunction, principal determinant of micro- and macrovascular dysfunction, shifts the vascular endothelial balance toward more vasoconstriction and inflammation with recruitment of immune cells and a pro-coagulant state [59].
In the lung in addition to type II alveolar cells and macrophages, ACE2 receptor is also widely expressed by microvascular endothelial cells (Fig. 1 ).
Fig. 1.
Putative mechanisms of SARS-CoV-2 infection within the alveolus. When SARS-CoV-2 infects the lower pulmonary airways, it can directly attack alveolar type II alveolar cells and resident macrophages, both expressing the ACE2 receptor. In response to viral infection, these cells produce various proinflammatory chemokines and cytokines. SARS-CoV-2 can also directly infect both capillary endothelial cell (increasing the permeability to plasma components at the infection site) and T cells (reducing the antiviral immune response). Stressed and necrotic cells release DAMPs and PAMPs mediators. These ligands interact with the RAGE, a highly expressed receptor in lung epithelial cells and stimulate downstream signalling that perpetuates an unfavourable proinflammatory state. Neutrophils can release NETs, which could damage endothelial cells. The hypercytokinemia attracts a greater number of monocytes-macrophages (the main sources of pro-inflammatory cytokines) and neutrophils from the bloodstream to the infection/inflammation site, to remove exudates. This massive cell infiltration into the alveolar or interstitial spaces causes a “cytokine storm” which promotes further cellular apoptosis and leads to further worsening of lung injury.
In different organs of COVID-19 patients, viral particles were found within dead endothelial cells associated with an accumulation of dead inflammatory cells, evidencing a direct viral attack and a diffuse host's inflammatory response in the vessel wall [30]. Viral replication within the alveolar cells causes apoptosis of epithelial cells, exposing the ACE2 receptor of the lung endothelial cells to the direct viral infection, triggering enhanced endothelial permeability and destruction of the local microcirculation (Fig. 1).
The recruitment of immune cells at the infection site can also cause a widespread endothelial dysfunction resulting in cell death such as apoptosis and pyroptosis [23] (Fig. 1).
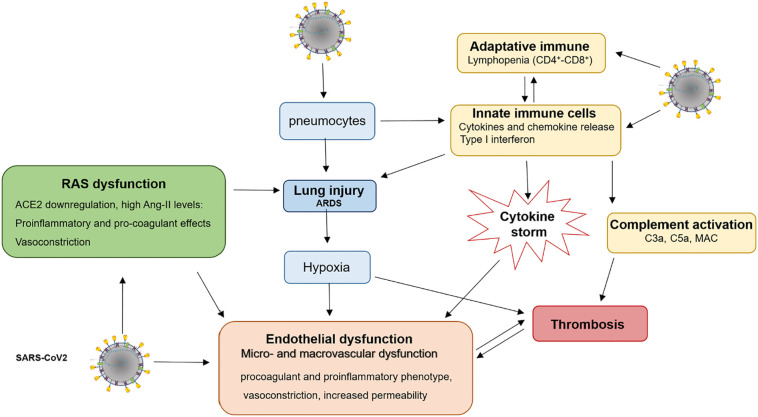
It is reasonable to assume that the endothelial dysfunction can be promoted both by direct action of SARS-CoV-2 virus to endothelial ACE2 interaction and by indirect mechanisms (see the following paragraph), as hypoxia, a hyper-inflammatory and immune dysregulation, resulting in CV collapse of severe COVID-19 (Fig. 2 ).
Fig. 2.
A comprehensive overview of SARS-CoV-2-induced endothelial injury and thrombotic complications. SARS-CoV2 infects a number of cell types, including type II alveolar cells, macrophages, T cells and endothelial cells, leading to hyperinflammation, hypoxia, apoptosis and imbalance of renin-angiotensin system. High levels of proinflammatory cytokines/chemokines can directly induce endothelial leak, cause cell apoptosis or also promote systemic inflammation and thrombosis. High levels of Ang-II switch endothelium to a proinflammatory and procoagulant phenotype. The ARDS- induced hypoxia can cause endothelial dysfunction by mitochondrial ROS generation, intracellular acidosis, cell signalling pathway activation, and can increase blood viscosity. Together, a dysregulated immune response, hypercytokinemia, imbalance of RAS, complement activation and hypoxemia induce an exacerbated endothelial dysfunction and thrombosis.
This assumption provides a rationale for anti-inflammatory drugs, ACE inhibitors, and statin therapies to counteract the endothelial injury, particularly in those patients with a pre-existing endothelial dysfunction due to comorbidities such as diabetes, hypertension and obesity, that make an individual more vulnerable to subsequent endotheliitis of SARS-CoV-2 infection.
5. Endothelial dysfunction indirectly caused by the SARS-CoV-2 infection
5.1. Injury induced by hypoxia
The main clinical manifestation of COVID-19 patients is an acute hypoxic respiratory failure that, in many cases, results in ARDS and needs for invasive mechanical ventilation [60,61]. Endothelial cells act as functional vascular oxygen sensors, through different cell PO2-mechanosensors, and adapt their metabolism to maintain ATP production. Hypoxemia induces endothelial cells to significant metabolic changes such as the reduction in cell ATP generation by oxidative phosphorylation and a shift from aerobic toward anaerobic catabolic metabolism [62]. The switch into hypoxic metabolism leads to the production of mitochondrial reactive oxygen species (ROS). Indeed, when PO2 is low, the mitochondrial respiratory chain works slower and an alteration of the cellular redox potential leads to an accumulation of electrons inducing ROS formation [63].
As above-mentioned, a viral consumption of ACE2 can lead to an accumulation of Ang-II with consequent activation of AT1 receptor that can cause not only a rapid vasoconstriction, vascular permeability, pulmonary edema and inflammation, but also enhanced ROS production. In fact, Ang-II is a classic prooxidant peptide that increases superoxide anion production through the activation of NAD(P)H oxidases in various vascular cell types, including endothelial cells, causing increased consumption of oxygen and energy [64]. Under physiological conditions, the superoxide dismutase catalyzes the dismutation of superoxide anion in H2O2 while when produced in excess, a significant amount of peroxide anion reacts with NO to produce peroxynitrite, leading to further cell injury. Therefore, hypoxia-induced endothelial injury may likely be secondary to increased Ang-II levels and associated pulmonary injury which in turn promote hypoxia.
During hypoxia, pyruvate, cannot contribute to the mitochondrial respiratory chain, therefore it is transformed into lactate, leading to intracellular acidosis [63]. Both intracellular oxidative stress and acidosis are harmful for cell vitality.
Similarly, anaerobic metabolism allows the heart to use lactic acid as an energy source by increasing the acidosis of cardiomyocytes inducing irreversible damage to cellular activities. The conditions of hypoxia and acidosis, which entail the loss of the integrity of the lysosomal membrane with the consequent release of enzymes, can also damage the adjacent cells [65].
Hypoxemia can also be detrimental through the hypoxia-induced influx of calcium ions that can also lead to damage and apoptosis of cardiomyocytes [66]. Therefore, the systemic viral infection associated with hypoxia in acute respiratory failure can impair myocardial oxygen demand, leading to acute myocardial injury.
5.2. Injury induced by cytokine storm and immune dysregulation: blood-clotting complications
COVID-19 illness exhibits three grades of increasing severity that correspond to distinct clinical features, potential therapy and clinical outcome [67]. A first mild stage with an early infection, characterized by mild symptoms and rapid activation of the innate immune response, namely from monocytes and macrophages. A second moderate stage characterized by pulmonary involvement without or with hypoxia, lymphopenia, increased inflammatory processes and a third stage with severe respiratory failure, septic shock and multiple organ dysfunction syndrome resulting in fatality in half of such cases [67]. This small subgroup of patients (5%) presents an extra-pulmonary systemic hyperinflammation with an elevated cytokine profile, lymphopenia and altered profile coagulative.
Chemokines and pro-inflammatory mediators are initially released in the area focal infected by pneumocytes and by local macrophages and dendritic cells that undergo the direct attack of the virus (Fig. 1). Subsequently, neutrophils and monocytes-macrophages (the major sources of pro-inflammatory cytokines) move from the bloodstream and flow to the infection/inflammation site, invading alveolar or interstitial spaces and sparking the so-called “cytokine storm” that causes further injury of epithelial and EC. An excessive and uncontrolled release of pro-inflammatory cytokines spreads all over the body through circulation rapidly provoking vascular permeability and leakage, activation of the coagulation, immune cell differentiation contributing to ARDS single or multiple organ failure, and eventually death [68,69].
The cytokines and chemokines associated with COVID-19 cytokine storm and disease severity are mainly tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, IL-7, IL-10, granulocyte-colony stimulating factor, interferon-γ inducible protein 10, monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1-α, platelet derived growth factor [68,[70], [71], [72]]. Serum SARS-CoV-2 RNA level was associated with elevated IL-6 concentration and poor prognosis suggesting that multiple organ dysfunction in severe COVID-19 patients could be at least partially caused by a direct attack from the virus [31].
It is critical to understand that aberrant generation of free radicals, the downstream product of cytokine storm, represents the primary cause of endothelial dysfunction, leading to direct damage to cells and multiple organs dysfunction. NO produced by endothelial nitric oxide synthase (eNOS) is a key determinant for vascular homeostasis, maintaining antiproliferative, antithrombotic and antiatherogenic phenotype. Reduction in NO bioavailability, resulting from reduced NO production and/or increased NO degradation by ROS, is a hallmark of endothelial dysfunction and thrombotic events. A decreased activity of ACE2, as observed in COVID-19, determines relative low levels of the metabolite Ang-(1–7), that in physiological conditions exerts its crucial antioxidative and vasoprotective role by a potent induction of NO [73]. Rabelo et al. demonstrated reduced eNOS (nitric oxide synthase) expression at both protein and mRNA level and reduced NO bioavailability in ACE2-deficient animals [64]. Moreover, NO inhibited the replication cycle of SARS-CoV and this could be the same for SARS-CoV2. On the other hand, dysregulation of NO signalling pathways provide a permissive cellular environment for viral entry and replication, as demonstrated by the treatment with NO donor compounds in SARS-CoV infection [74].
Mild/moderate COVID-19 is associated with potent type-I Interferon (IFN)-mediated antiviral response while it is reduced in more severe patients [75]. The role of this cytokine has been characterized as triggering antiviral states in cells and potentiating adaptive immune responses. In mild/moderate infection, a fast antiviral response with type-I IFN consents a quick decrease of viral load, preventing T-cell exhaustion and hyperinflammation. In severe COVID-19, a poor and late antiviral response results in high lung hypercytokinemia, weakened T-cell resistance, and acute clinical worsening. However, the kinetics and intensity of the type-I IFN response, during SARS-CoV-2 infection, that could be linked to clinical outcome, remains to be elucidated.
Elevated levels of blood neutrophils predict severe respiratory disease and worse outcomes in COVID-19 patients [68,76]. Moreover, many patients with COVID-19 showed high levels of neutrophil extracellular traps (NETs), networks of extracellular chromatin and histones that are released by neutrophils to trap pathogens [77]. NETs represent an important strategy to immobilize and kill invading microorganisms, but if not properly regulated, have potential to induce endothelial activation and dysfunction to initiate and propagate inflammation in the vessel wall, and, when formed intravascularly, drive thrombotic events in macro and microvascular vessels, leading to organ damage [78,79] (Fig. 1). Chromatin and histones exert cytotoxic effects on endothelial cells [80] and there is experimental evidence supporting a role for histones in ARDS and sepsis [81].
Severe COVID-19 is characterized by low levels of helper T cells, regulatory T cells and natural killer cells, i.e. lymphopenia and depression of antiviral immunity [82,83]. In particular, CD4+ T cells and CD8+ T cells were slightly lower in moderate cases and dramatically decreased in severe COVID-19, representing a critical factor associated with lung injury severity [82]. Although the mechanism underlying lymphopenia and impaired antiviral responses observed in the severe cases of COVID-19 needs to be better clarified, recent data suggest that SARS-CoV-2 can directly infect T cells through a receptor-dependent manner [84]. However, T cells have a scant expression of ACE2, suggesting either another receptor or a high spike protein affinity for ACE2. The infection of T cells is unproductive, because SARS-CoV-2 cannot replicate within T cells but rather induces a cytotoxic action and death [84]. Therefore, an increased apoptotic cell death, cytokine insults, the advanced age, and the presence of CV comorbidities could indirectly contribute to lymphopenia [84,85].
The complement system is a key player of the innate immune response against pathogens but its excessive activation may contribute to the inflammatory response [86]. A study demonstrated that complement C3-deficient mice infected with SARS-CoV show ameliorating lung injury, reduced neutrophilia and systemic inflammation, suggesting that complement inhibitors represent an important strategy to counteract lung complications in SARS-CoV and SARS-CoV-2 infection [87]. Data on the role of complement activation in the development of SARS-CoV-2-associated ARDS are scarce. A preprint study reported that lung biopsy samples from patients with severe COVID-19 revealed complement activation, characterized by C3a generation and C3-fragment deposition [88]. Recently, a report showed beneficial effects of C3 inhibitor AMY-101 on a patient with severe COVID-19 pneumonia, through a reduction of biomarkers of systemic hyperinflammation and improved lung function [89].
Stressed and necrotic cells release danger associated molecular pattern (DAMPs) molecules, such as high mobility group protein B1 (HMGB1), and pathogen associated molecular patterns (PAMPs) molecules, to alarm the environment about the loss of cellular homeostasis [90]. HMGB1, a chromatin-associated non-histone protein, activates cells through binding to multiple cell-surface receptors, including TLR4 receptors, amplifying the pro-inflammatory cytokine release [90].
Extracellular HMGB1, in conjunction with extracellular DNA, RNA and other DAMP or PAMP molecules are endocytosed via the receptor for advanced glycation endproducts (RAGE) [91,92], constitutively and abundantly expressed in the lungs [93], and transported to the endolysosomal system, to be destroyed in the lysosomes (Fig. 1). However, the lysosomal system is inefficient in the presence of high levels of HMGB1 [90], so the HMGB1/RAGE axis can initiate and perpetuate an unfavourable proinflammatory state that compromises the integrity of large tissue areas [94]. Importantly, the HMGB1-RAGE axis has been shown to induce neutrophil-mediated lesion amplification following necrosis [90], and this can be of particular significance for severe COVID-19 pneumonia.
Recently, there has been an unusual increase in the number of children suffering from multisystem inflammatory syndromes often similar to Kawasaki disease [95,96], a rare pathology that is characterized by systemic vasculitis, i.e. inflammation of the blood vessels [97]. The most serious complication are coronary artery aneurysms, which can lead to death from myocardial infarction even at a young age. Since the affected subjects had been infected by SARS-CoV-2, scientists suspect that Kawasaki disease may be triggered by the virus that causes an abnormal immune response with secondary vasculitis [95,96].
Hyperinflammation, immune activation and hypoxia may play a decisive role in endothelial dysfunction, associated disseminated intravascular coagulation (DIC) and CV risk factors may increase the susceptibility to microvascular injury and microthrombus formation predisposing COVID19 patients to both venous and arterial thromboembolic disease [1,22,98]. Studies suggested that blood clots appear in 20% to 30% of critically ill COVID-19 patients [61,99]. Abnormal coagulation parameters, as elevated fibrin marker (e.g. D-dimer, fibrin degradation products), and prolongation of the coagulation times evidence the coexistence of coagulation activation and hyperfibrinolysis in COVID-19 patients [100]. A disarray of the coagulation and fibrinolytic system was noted in the 71.4% of patients who did not survive the infection [100]. This defective procoagulant–anticoagulant balance would predispose to hypercoagulable state, meeting criteria for DIC. In presence of systemic infection, DIC is characterized by over-inflammatory response, strictly linked to endothelial proinflammatory and prothrombotic phenotype. Endothelial activation induced by infection and by cytokine storm, as in COVID-19, results in tissue factor (TF) expression, trigger of the extrinsic pathway of coagulation [101], in excess thrombin generation and fibrinolysis shutdown, due to decreased levels of endogenous anticoagulant and increased levels of Plasminogen Activator Inhibitor-1 (PAI-1) [102]. This complex picture could indicate a hypercoagulable state in patients with severe COVID-19 [103,104]. During the progression of COVID-19, inflammation could lead to an uncontrolled activation of Xa factor, point of convergence of extrinsic and intrinsic pathways of coagulation, and thrombin, protease responsible of fibrin clot formation and stabilization. In addition to hemostatic properties, factor Xa and thrombin promote in turn cell processes as inflammation and angiogenesis via proteinase-activated receptors expressed on vascular cells [105]. Factor Xa proven to induce IL-6 expression and cell proliferation in lung fibroblasts, underlying as an uncontrolled activation of the coagulation cascade may contribute to lung diseases [106]. In COVID-19, factor Xa could have a crucial role, as it could facilitate SARS-CoV-2 cell invasiveness by its proteolytic cleavage of spike protein, as previously demonstrate for SARS-CoV [107]. Interestingly, some COVID-19 patients showed elevated circulating levels of von Willebrand factor (vWF) antigen, and factor VIII indicating endothelial stimulation and damage [108,109]. The vWF, a multimeric glycoprotein physiologically stored inside Weibel-Palade bodies of endothelial cells, may contribute to tissue injury and organ failure in thrombo-inflammatory disorders mediating platelet adhesion to damaged endothelium, modulating vascular permeability and edema formation, and promoting inflammation and complement activation [110]. In normal conditions, vWF is cleaved by protease ADAMTS13, but in severe inflammatory states, upon systemic infection, a deficiency of ADAMTS13 lead to increased levels of active vVW [111]. Currently, there are no data on ADAMTS13, PAI-1 and TF concentrations in patients with severe COVID-19 infection.
In association with lymphopenia and elevated D-Dimer levels, some COVID-19 patients showed thrombocytopenia, a condition characterized by a low platelet count associated with increased risk of severe disease and mortality in patients with COVID-19 [1,68,100,112]. SARS-CoV-2 may inhibit primary platelet production, attacking bone marrow cells via ACE2 directly or indirectly by the cytokine storm, or lead platelet destruction increasing autoantibody and immune complex formation. In addition, damaged lung tissues and pulmonary endothelial cells may induce platelet activation and aggregation in the lungs, resulting in platelet consumption and intravascular microthrombi formation [113,114].
Some COVID-19 patients show a systemic procoagulant state with blood clots and extensive deposition of factors of alternative pathway and lectin pathway of complement in smallest vessels of lung and skin samples emphasizing the potential role of complement in microvascular injury [115]. In addition, the hypoxia in severe COVID-19 may stimulate thrombosis not only increasing blood viscosity, but also hypoxia-inducible transcription factors whose target genes include several factors that regulate thrombus formation, such as TF and PAI-1 [116].
The RAAS system is intrinsically linked to the coagulation cascade. Elevated levels of Ang-II in COVID19 patients [83] would exacerbate endothelial dysfunction and sustain the role of the endothelium in thrombotic complications in COVID-19. It is know that Ang II increase endothelial expression of TF and PAI-1 e and endothelial release of complement factors, contributing to hypercoagulable state [117]. This may explain why fibrin deposits observed in the lung alveoli of SARS and COVID-19 victims [26,87].
6. Conclusion
Endothelial dysfunction concerns a systemic condition in which the endothelium loses its physiological properties such as the control of vascular tone, haemostasis, vascular permeability, recruitment of neutrophils, hormonal traffic, including the tendency to promote vasodilation, fibrinolysis, and platelet anti-aggregation.
The pathophysiology of endothelial function plays a significant role in the development and progression of CVD and can be significantly disrupted and exacerbated by SARS-CoV-2 infection. Pre-existing endothelial dysfunction is the common denominator among previous comorbidities such as hypertension, diabetes, obesity, CVD and aging˗ of people with an increased risk of severe COVID-19.
Endothelial cells can be directly affected by the viral infection but they can also undergo the cytokine tsunami triggered during the viral infection. Diffuse endothelial inflammation can be the consequence of a dysregulated immune response that results in excessive activation of the endothelium that becomes a real battleground of SARS-CoV-2. Hypercytokinemia can directly induce endothelial leak through the interruption of cell-cell junctions, cause cell apoptosis or also induce the expression of proinflammatory, adhesion and procoagulant molecules that promote systemic inflammation, leukocyte recruitment and a procoagulant state. It is known that microthrombi begin to form early in the lungs of severe COVID-19, possibly because of the interplay between inflammatory factors and coagulation.
Since mortality from COVID-19 remains high, especially among the elderly people, new therapeutic strategies that target the lung microvascular endothelium in conjunction with specific antiviral administration could represent a promising therapeutic strategy to reduce fatality rate.
Declaration of competing interest
None.
Acknowledgements
None.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(e8):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.WHO . 2020. Coronavirus Disease (COVID-19). Situation Report – 176. July 14 2020. [Google Scholar]
- 6.Lin Y.F., Duan Q., Zhou Y., Yuan T., Li P., Fitzpatrick T., et al. Spread and impact of COVID-19 in China: a systematic review and synthesis of predictions from transmission-dynamic models. Front. Med. (Lausanne) 2020;7:321. doi: 10.3389/fmed.2020.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo G., Ye L., Pan K., Chen Y., Xing D., Yan K., et al. New insights of emerging SARS-CoV-2: epidemiology, etiology, clinical features, clinical treatment, and prevention. Front. Cell. Dev. Biol. 2020;8:410. doi: 10.3389/fcell.2020.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 13.Cowan L.T., Lutsey P.L., Pankow J.S., Matsushita K., Ishigami J., Lakshminarayan K. Inpatient and outpatient infection as a trigger of cardiovascular disease: the ARIC study. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu C.M., Wong R.S., Wu E.B., Kong S.L., Wong J., Yip G.W., et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad. Med. J. 2006;82:140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madjid M., Miller C.C., Zarubaev V.V., Marinich I.G., Kiselev O.I., Lobzin Y.V., et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur. Heart J. 2007;28:1205–1210. doi: 10.1093/eurheartj/ehm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhainaut J.F., Claessens Y.E., Janes J., Nelson D.R. Underlying disorders and their impact on the host response to infection. Clin. Infect. Dis. 2005;41(Suppl. 7):S481–S489. doi: 10.1086/432001. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y., Sun J., Dai Z., Deng H., Li X., Huang Q., et al. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su H., Yang M., Wan C., Yi L.X., Tang F., Zhu H.Y., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020 doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shamshirian A., Heydari K., Alizadeh-Navaei R., Moosazadeh M., Abrotan S., Hessami A. 2020. Cardiovascular Diseases and COVID-19 Mortality and Intensive Care Unit Admission: A Systematic Review and Meta-analysis. medRxiv. [DOI] [Google Scholar]
- 20.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 21.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12(8) doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020 doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox S.E., Akmatbekov A., Harbert J.L., Guang Li J., Brown J.Q., Vander Heide R.S. 2020. Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 27.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020:217. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C., Xie J., Zhao L., Fei X., Zhang H., Tan Y., et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. 2020;57 doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020 doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L., et al. The presence of SARS-CoV-2 RNA in feces of COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- 33.Ling Y., Xu S.B., Lin Y.X., Tian D., Zhu Z.Q., Dai F.H., et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann. Intern. Med. 2020 doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020 doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicin L., Abplanalp W.T., Mellentin H., Kattih B., Tombor L., John D., et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H., Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit. Care. 2017;21:305. doi: 10.1186/s13054-017-1882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020 doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reudelhuber T.L. The renin-angiotensin system: peptides and enzymes beyond angiotensin II. Curr. Opin. Nephrol. Hypertens. 2005;14:155–159. doi: 10.1097/00041552-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Burrell L.M., Johnston C.I., Tikellis C., Cooper M.E. ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol. Metab. 2004;15:166–169. doi: 10.1016/j.tem.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohn J.N. Reducing cardiovascular risk by blockade of the renin-angiotensin-aldosterone system. Adv. Ther. 2007;24:1290–1304. doi: 10.1007/BF02877776. [DOI] [PubMed] [Google Scholar]
- 45.Sukumaran V., Veeraveedu P.T., Gurusamy N., Lakshmanan A.P., Yamaguchi K., Ma M., et al. Olmesartan attenuates the development of heart failure after experimental autoimmune myocarditis in rats through the modulation of ANG 1-7 mas receptor. Mol. Cell. Endocrinol. 2012;351:208–219. doi: 10.1016/j.mce.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 47.Iwanami J., Mogi M., Tsukuda K., Wang X.L., Nakaoka H., Ohshima K., et al. Role of angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis in the hypotensive effect of azilsartan. Hypertens. Res. 2014;37:616–620. doi: 10.1038/hr.2014.49. [DOI] [PubMed] [Google Scholar]
- 48.Khashkhusha T.R., Chan J.S.K., Harky A. ACE inhibitors and COVID-19: we don’t know yet. J. Card. Surg. 2020 doi: 10.1111/jocs.14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soria Arcos F., Romero-Puche A., Vicente Vera T. Controversy regarding ACE inhibitors/ARBs in Covid-19. Rev. Esp. Cardiol. 2020 doi: 10.1016/j.recesp.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sriram K., Insel P.A. Risks of ACE inhibitor and ARB usage in COVID-19: evaluating the evidence. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milne S., Yang C.X., Timens W., Bosse Y., Sin D.D. SARS-CoV-2 receptor ACE2 gene expression and RAAS inhibitors. Lancet Respir. Med. 2020;8 doi: 10.1016/S2213-2600(20)30224-1. e50-e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meng J., Xiao G., Zhang J., He X., Ou M., Bi J., et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehta N., Kalra A., Nowacki A.S., Anjewierden S., Han Z., Bhat P., et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghosal S., Mukherjee J.J., Sinha B., Gangopadhyay K.K. 2020. The Effect of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers on Death and Severity of Disease in Patients With Coronavirus Disease 2019 (COVID-19): A Meta-analysis. medRxiv. [DOI] [Google Scholar]
- 58.Godo S., Shimokawa H. Endothelial functions. Arterioscler. Thromb. Vasc. Biol. 2017;37 doi: 10.1161/ATVBAHA.117.309813. e108-e14. [DOI] [PubMed] [Google Scholar]
- 59.Rajendran P., Rengarajan T., Thangavel J., Nishigaki Y., Sakthisekaran D., Sethi G., et al. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He J., Wu B., Chen Y., Tang J., Liu Q., Zhou S., et al. Characteristic electrocardiographic manifestations in patients with COVID-19. Can. J. Cardiol. 2020 doi: 10.1016/j.cjca.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koziel A., Jarmuszkiewicz W. Hypoxia and aerobic metabolism adaptations of human endothelial cells. Pflugers Arch. 2017;469:815–827. doi: 10.1007/s00424-017-1935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rabelo L.A., Todiras M., Nunes-Souza V., Qadri F., Szijarto I.A., Gollasch M., et al. Genetic deletion of ACE2 induces vascular dysfunction in C57BL/6 mice: role of nitric oxide imbalance and oxidative stress. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCarthy C.P., Januzzi J.L., Jr., Gaggin H.K. Type 2 myocardial infarction - an evolving entity. Circ. J. 2018;82:309–315. doi: 10.1253/circj.CJ-17-1399. [DOI] [PubMed] [Google Scholar]
- 66.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020 doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020:34. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 71.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heitsch H., Brovkovych S., Malinski T., Wiemer G. Angiotensin-(1-7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension. 2001;37:72–76. doi: 10.1161/01.hyp.37.1.72. [DOI] [PubMed] [Google Scholar]
- 74.Keyaerts E., Vijgen L., Chen L., Maes P., Hedenstierna G., Van Ranst M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004;8:223–226. doi: 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mantlo E., Bukreyeva N., Maruyama J., Paessler S., Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antivir. Res. 2020;179:104811. doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gould T.J., Vu T.T., Swystun L.L., Dwivedi D.J., Mai S.H., Weitz J.I., et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler. Thromb. Vasc. Biol. 2014;34:1977–1984. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 79.Ma A.C., Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J. Thromb. Haemost. 2008;6:415–420. doi: 10.1111/j.1538-7836.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- 80.Saffarzadeh M., Juenemann C., Queisser M.A., Lochnit G., Barreto G., Galuska S.P., et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wygrecka M., Kosanovic D., Wujak L., Reppe K., Henneke I., Frey H., et al. Antihistone properties of C1 esterase inhibitor protect against lung injury. Am. J. Respir. Crit. Care Med. 2017;196:186–199. doi: 10.1164/rccm.201604-0712OC. [DOI] [PubMed] [Google Scholar]
- 82.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang X., Xu W., Hu G., Xia S., Sun Z., Liu Z., et al. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell. Mol. Immunol. 2020 doi: 10.1038/s41423-020-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiong T.Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., et al. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery D., Jensen K., Leist S.R., et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio. 2018;9 doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao T., Hu M., Zhang X., Li H., Zhu L., Liu H., et al. 2020. Highly Pathogenic Coronavirus N Protein Aggravates Lung Injury by MASP-2-mediated Complement Over-activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mastaglio S., Ruggeri A., Risitano A.M., Angelillo P., Yancopoulou D., Mastellos D.C., et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huebener P., Pradere J.P., Hernandez C., Gwak G.Y., Caviglia J.M., Mu X., et al. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J. Clin. Invest. 2019;130:1802. doi: 10.1172/JCI126976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Basta G., Del Turco S., Navarra T., Lee W.M., G. Acute Liver Failure Study Circulating levels of soluble receptor for advanced glycation end products and ligands of the receptor for advanced glycation end products in patients with acute liver failure. Liver Transpl. 2015;21:847–854. doi: 10.1002/lt.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miniati M., Monti S., Basta G., Cocci F., Fornai E., Bottai M. Soluble receptor for advanced glycation end products in COPD: relationship with emphysema and chronic cor pulmonale: a case-control study. Respir. Res. 2011;12:37. doi: 10.1186/1465-9921-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bodine B.G., Bennion B.G., Leatham E., Jimenez F.R., Wright A.J., Jergensen Z.R., et al. Conditionally induced RAGE expression by proximal airway epithelial cells in transgenic mice causes lung inflammation. Respir. Res. 2014;15:133. doi: 10.1186/s12931-014-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andersson U., Ottestad W., Tracey K.J. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19? Mol. Med. 2020;26:42. doi: 10.1186/s10020-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harahsheh A.S., Dahdah N., Newburger J.W., Portman M.A., Piram M., Tulloh R., et al. Missed or delayed diagnosis of Kawasaki disease during the 2019 novel coronavirus disease (COVID-19) pandemic. J. Pediatr. 2020 doi: 10.1016/j.jpeds.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Bradley Segal J., et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp. Pediatr. 2020 doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 97.Capittini C., Emmi G., Mannarino S., Bossi G., Dellepiane R.M., Salice P., et al. An immune-molecular hypothesis supporting infectious aetiopathogenesis of Kawasaki disease in children. Eur. J. Immunol. 2018;48:543–545. doi: 10.1002/eji.201747226. [DOI] [PubMed] [Google Scholar]
- 98.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 100.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Del Turco S., Basta G., Lazzerini G., Evangelista M., Rainaldi G., Tanganelli P., et al. Parallel decrease of tissue factor surface exposure and increase of tissue factor microparticle release by the n-3 fatty acid docosahexaenoate in endothelial cells. Thromb. Haemost. 2007;98:210–219. [PubMed] [Google Scholar]
- 102.Beristain-Covarrubias N., Perez-Toledo M., Thomas M.R., Henderson I.R., Watson S.P., Cunningham A.F. Understanding infection-induced thrombosis: lessons learned from animal models. Front. Immunol. 2019;10:2569. doi: 10.3389/fimmu.2019.02569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Levi M., van der Poll T. Coagulation and sepsis. Thromb. Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 104.Schmitt F.C.F., Manolov V., Morgenstern J., Fleming T., Heitmeier S., Uhle F., et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann. Intensive Care. 2019;9(19) doi: 10.1186/s13613-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spronk H.M., de Jong A.M., Crijns H.J., Schotten U., Van Gelder I.C., Ten Cate H. Pleiotropic effects of factor Xa and thrombin: what to expect from novel anticoagulants. Cardiovasc. Res. 2014;101:344–351. doi: 10.1093/cvr/cvt343. [DOI] [PubMed] [Google Scholar]
- 106.Scotton C.J., Krupiczojc M.A., Konigshoff M., Mercer P.F., Lee Y.C., Kaminski N., et al. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J. Clin. Invest. 2009;119:2550–2563. doi: 10.1172/JCI33288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Du L., Kao R.Y., Zhou Y., He Y., Zhao G., Wong C., et al. Cleavage of spike protein of SARS coronavirus by protease factor Xa is associated with viral infectivity. Biochem. Biophys. Res. Commun. 2007;359:174–179. doi: 10.1016/j.bbrc.2007.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Escher R., Breakey N., Lammle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gragnano F., Sperlongano S., Golia E., Natale F., Bianchi R., Crisci M., et al. The role of von Willebrand factor in vascular inflammation: from pathogenesis to targeted therapy. Mediat. Inflamm. 2017;2017:5620314. doi: 10.1155/2017/5620314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Levi M., Scully M., Singer M. The role of ADAMTS-13 in the coagulopathy of sepsis. J. Thromb. Haemost. 2018;16:646–651. doi: 10.1111/jth.13953. [DOI] [PubMed] [Google Scholar]
- 112.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu P., Zhou Q., Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 2020;99:1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Y., Zeng X., Jiao Y., Li Z., Liu Q., Ye J., et al. Mechanisms involved in the development of thrombocytopenia in patients with COVID-19. Thromb. Res. 2020;193:110–115. doi: 10.1016/j.thromres.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020 doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gupta N., Zhao Y.Y., Evans C.E. The stimulation of thrombosis by hypoxia. Thromb. Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 117.Watanabe T., Barker T.A., Berk B.C. Angiotensin II and the endothelium: diverse signals and effects. Hypertension. 2005;45:163–169. doi: 10.1161/01.HYP.0000153321.13792.b9. [DOI] [PubMed] [Google Scholar]