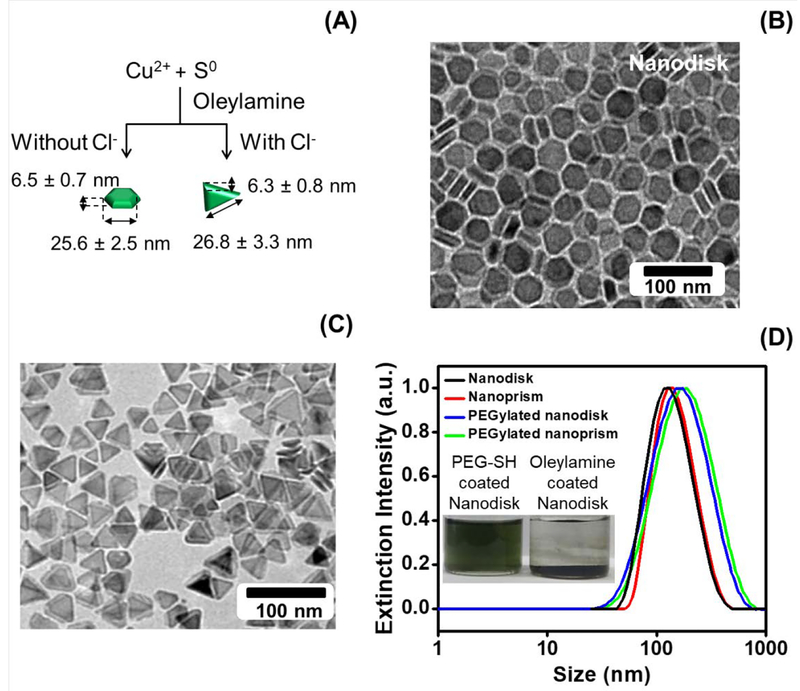
Figure 1: Physical and chemical characterization of CuS nanodisks and nanoprism.
Panel A depicts the synthesis of CuS nanodisks and nanoprisms via a solvent-based and seed/halide ion-mediated process. Panels B and C are the TEM images of the CuS nanodisks and nanoprisms, respectively. The average diameter and thickness of CuS nanodisk was 25.6 nm and 6.5 nm. The nanoprism was 26.8 wide and 6.3 nm thick. Panel D shows the hydrodynamic radius of the oleyamine-coated nanodisks (85.3 nm) and nanoprisms (91.9 nm) in chloroform. The hydrodynamic radius of the PEGylated nanodisks and nanoprism dissolved in water increased to 125.4 and 131.7 nm, respectively. While the oleynamine-coated CuS nanodisks precipitate in water, PEGylation stabilizes the nanodisk in water (inset). The size difference between TEM and DLS is caused by the DLS algorithm that is optimized for spherical particles and is a potential error source.