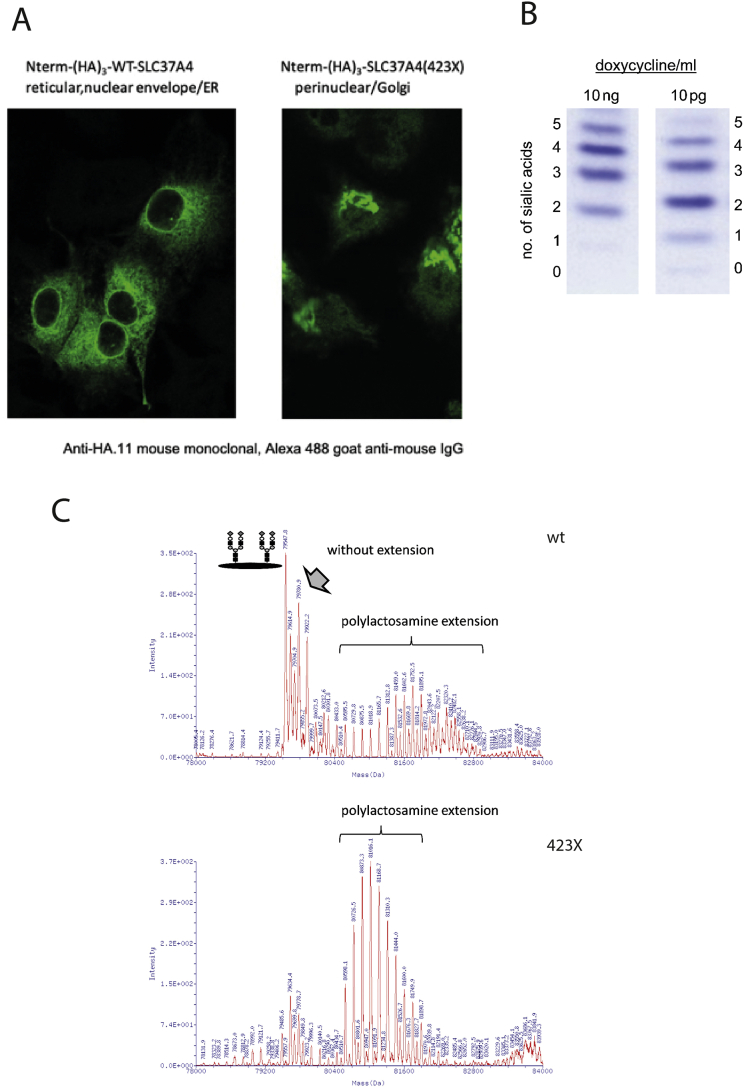
Fig. 4.
A. SLC37A4-423X variant mislocalizes from the ER to the Golgi. Human wild-type SLC37A4 or the mutated SLC37A4 (423X), both tagged with an N-terminal triple repeat of a hemagglutinin epitope (HA3-SLC37A4), were expressed in HepG2 cells. The wildtype protein resides in the ER of HepG2 tetoff cells; by contrast, the truncated version is present within the Golgi apparatus. Intracellular localization was confirmed by costaining with antibodies for GRP78 (ER), syntaxin 5 (Golgi) and TGN38 (trans Golgi network) (not shown). B. IEF of transferrin from HepG2 cells expressing SLC37A4-R423*. Lowering the doxycycline concentration increases the expression of the mutant protein leading to an increase in hypoglycosylation. C. Mass spectrometry of transferrin glycoforms isolated from HepG2 cells transfected with either wt or truncated R423* SLC37A4. Transfection with the mutated transporter reduced normal glycosylation and increased the amount of polylactosamines. Polylactosamine are galactose/N-acetylglucosamine repeats of different lengths.