Abstract
Tuber luomae, a new truffle species known only from the Pacific Northwest, USA, is distinguished by spiny, non-reticulate spores and a two-layered peridium — the outermost layer (pellis) consists of inflated, globose to subpolygonal cells and the inner (subpellis) of narrow hyphae. ITS sequence analyses show that it has phylogenetic affinity to other Tuber species in the Rufum clade. The only other members of the Rufum clade with a strongly developed peridiopellis of large, inflated cells are the southern European T. malacodermum and T. pustulatum and the northern Mexican T. theleascum. We find it interesting that this peridial structure that is uncommon in the Rufum clade has been found in geographically disjunct species.
Keywords: new taxon, peridial structure, Pezizales, phylogeny, Rufum clade, truffle
INTRODUCTION
In 1980 Dr. Dan Luoma collected six Tuber ascomata on Orcas Island of the San Juan Islands, located in the straits separating coastal northwestern Washington (USA) from southern Vancouver Island (Canada). Microscopic examination revealed that although this collection has spiny spores and macroscopic characteristics that place it within the Rufum clade (Bonito et al. 2010), it is distinguished by a pellis of subglobose to globose or subpolyhedral cells up to 35 μm broad. Three other Tuber collections with the same morphological features have been discovered. Two of the collections were in northwestern Oregon, consisting of one ascoma in Benton County in 1962 and two ascomata in Clackamas County in 1995; one collection in southwestern Oregon consisted of one ascoma in Jackson County in 2012. We here describe this new species, Tuber luomae, and use ITS rDNA sequence data to place it within the Rufum clade of the genus Tuber.
MATERIALS AND METHODS
Specimens
Spiny-spored Tuber species, collected by raking (Weber et al. 1997) during the past four decades, were examined in this study. When possible, data on color and morphology of fresh ascomata were recorded and the specimens photographed prior to air-drying. Sections of the type specimen of Tuber luomae were paraffin-embedded, sectioned by microtome, and stained with Safranin O/ Fast-green (Johansen 1940). The other specimens were rehydrated in water and characterized anatomically by light microscopy. Spore measurements exclude the surface ornamentation of spines.
Phylogenetic analysis
Collections examined in this study and analyzed by molecular methods are presented in Table 1 along with their collection information and GenBank accession numbers (the Benton County collection had been preserved in an ethanol-formalin solution that had dried; reliable sequences were not obtainable).
Table 1.
Collections examined in this study and analyzed by molecular methods along with their collection information and GenBank accession numbers (the Benton County collection had been preserved in an ethanol-formalin solution that had dried; reliable sequences were not obtainable).
| Species | Collection ID1 | Origin | GenBank Accesion No. |
|---|---|---|---|
| Tuber candidum | SOC 727 | USA: Oregon | AY830856 |
| Tuber ferrugineum | n/a | n/a | AF132506 |
| Tuber huidongense | IFS Y. Wang 89924 | China | DQ478632 |
| Tuber indicum | Tind-my02 | China | DQ329365 |
| Tuber liaotongense | Tsp-hr02 | China | DQ478645 |
| Tuber luomae | *JT6003 | USA: Washington | FJ809887 |
| RB12-139 | USA: Oregon | MH142475 | |
| JT17457 | USA: Oregon | MH142474 | |
| Tuber lyonii | JT5665 | USA: Texas | FJ809883 |
| Tuber melanosporum | 1015 | Israel | AF167096 |
| Tuber melosporum | AH31737 | Spain | JX402095 |
| Tuber nitidum | BM105 | Spain | FJ809885 |
| Tuber pustulatum | JT32319 | Spain | FJ809889 |
| AQUI 9725 | Spain | MK211278 | |
| Tuber quercicola | SOC 733 | USA: Oregon | AY918957 |
| Tuber regimontanum | *ITC909 | Mexico | EU375838 |
| Tuber rufum | BOLO1506-1 | Italy | AY112894 |
| Tuber rufum var. lucidum | AZ2097 | Italy | FJ809888 |
| Tuber spinoreticulatum | *Uecker188 | USA: Maryland | FJ748913 |
| Tuber taiyuanense | IFS Y. Wang 610 | China | DQ478637 |
| Tuber theleascum | AQUI 9729 | Mexico | MK211283 |
| Tuber wenchuanense | FL-2013d strain HMAS 60239 | China | JX267044 |
1 Asterisk (*) denotes type collection.
Two procedures were used for molecular analysis. In the first, prior to DNA extraction, a small piece of previously unexposed glebal tissue was removed from the holotype (OSC 148707) collection with a sterile razor blade and pulverized with the aid of sterile sand, cubic zirconia beads and a Mini-Beadbeater (Biospec Products, Bartlesville, OK). DNA was extracted with 24:1 chloroform:isoamyl alcohol and precipitated with isopropanol. The internal transcribed spacer region (ITS1, 5.8S nrDNA, and ITS2) and part of the nuclear ribosomal large subunit (LSU) locus were amplified with the universal fungal primer sets ITS5 – ITS4 and LROR – LR5 (Vilgalys & Hester 1990, White et al. 1990, Bertini et al. 1999). The PCR cocktail and protocol followed that of Healy et al. (2009). PCR products were stained with 1× SYBR Safe (Invitrogen, Carlsbad, CA) and visualized on a 1.0 % agarose gel buffered with TAE buffer. Gel electrophoresis products were viewed on a GelDoc XR imager (Bio-Rad Laboratories, Inc., Hercules, CA). Qiagen Quick-Clean columns were used to clean PCR products prior to sequencing.
A second set of molecular methods was applied to the paratype specimens from Clackamas (OSC 148706) and Jackson (OSC 151373) counties; the Sigma Extract-N-Amp™ (Sigma-Aldrich, St. Louis, MO) protocol was followed by use of 15 μL of extract solution and 30 μL of dilution solution. PCR was performed with the universal primer set ITS1-F – ITS4 (White et al. 1990, Gardes & Bruns 1993) or the reverse primer ITS4Tuber, which was designed to improve specificity for Tuber (CTC GAC TCG TAG AAG ACA CT, Bonito unpublished). PCR products were stained with ethidium bromide after gel electrophoresis. PCR products were cleaned with ExoSAP-IT® (Affymetrix, Santa Clara, CA) prior to sequencing.
Sanger sequencing was performed in both directions using a BigDye Terminator v. 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) with ITS1-F, ITS5 or LROR (forward) and ITS4, ITS4Tuber or LR5 (reverse). DNA sequences were determined on an ABI PRISM 3700 DNA Analyzer (Applied Biosystems, Foster City, CA). Forward and reverse sequences were assembled and Sequencher v. 4.0 (Gene Codes, Ann Arbor, MI) was used to manually edit sequences and ambiguous regions at the ends were removed. Both ITS and LSU sequences were queried against the NCBI public database GenBank with the BLASTN algorithm for comparison with other sequences and to verify that the sequences belonged to Tuber.
DNA sequences were aligned manually for phylogenetic analysis with MacClade v. 4.0 (Maddison & Maddison 2002) and ambiguously aligned regions were excluded. Phylogenetic inference was conducted on the ITS alignment with RAxML computed through the CIPRES web portal (www.phylo.org) with a GTR model nucleotide substitution, and 1 000 bootstrap iterations. Outgroup taxa belonging to the Melanosporum clade were used because they are known to comprise the phylogenetic sister group to the Rufum clade within the genus Tuber (Bonito et al. 2010, 2013). Sequences produced with these procedures were deposited in GenBank under accession numbers FJ809887, MH142474 and MH142475, as was an LSU sequence not used in the alignment (FJ809812). Sequence alignments were deposited at TreeBASE under the accession number 22992.
RESULTS
Taxonomy
The description of Tuber luomae is based on the holotype. The three paratypes were carefully examined and did not differ in any significant details.
Tuber luomae Trappe, Eberhart, Piña Páez & Bonito, sp. nov. MycoBank MB807402. Fig. 1.
Fig. 1.
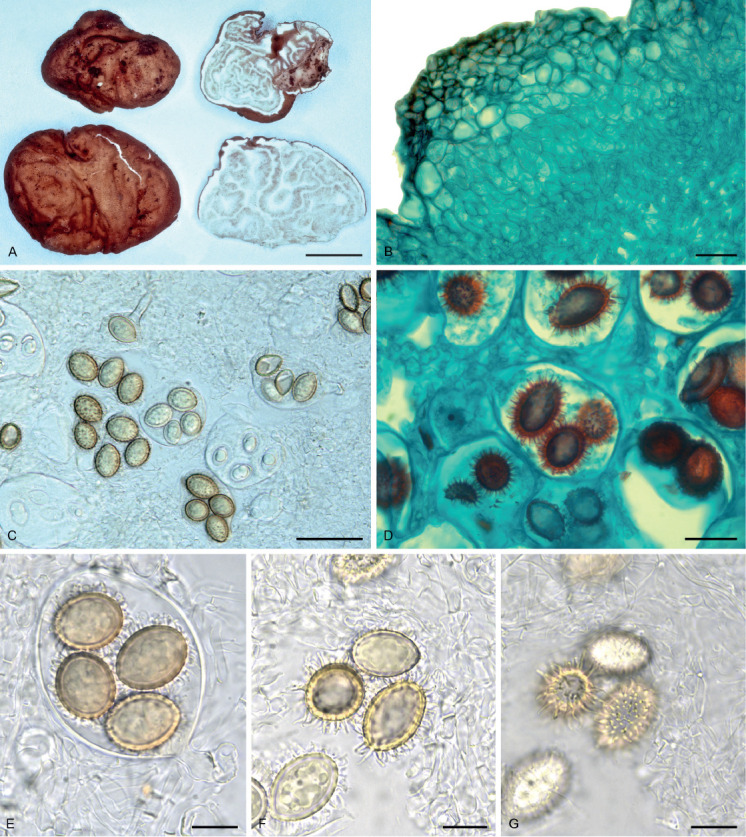
Morphological characters of Tuber luomae (Holotype, OSC 148707). A. Ascocarp, surface and cross-section view. B. Outer peridium in cross section composed of inflated cells with inner peridium abruptly differentiated as interwoven hyphae, stained with Safranin O/ Fast-green. C. One- to four-spored asci. D. Spiny spores stained with Safranin O/ Fast-green. E. Four-spored ascus with distinctive basal hyphal stem. F. Spore ornamentation, highlighting the spines. G. Spores at two focal depths illustrating the spines and lack of connecting reticulum. Scale bars: A = 10 mm; B, D = 20 μm; C = 40 μm; E–G = 15 μm.
Etymology: In honor of distinguished mycologist Dr. Dan Luoma, collector of the holotype, for his many contributions to mycology.
Typus: USA, Washington, San Juan Co., Orcas Island, Camp Orkila, elev. 20 m, 31 Oct. 1980, D. Luoma, Trappe 6003 (holotype OSC 148707; ITS GenBank FJ809887, LSU GenBank FJ809812).
Description: Ascomata when dried 20–25 × 25–30 mm, irregular, the surface even or with prominent wrinkles. Peridium light orange brown, minutely verrucose with a reddish-brown pellis over a pallid subpellis. Gleba with light brown fertile tissue marbled with prominent white veins. Odor in the field mildly acrid. Peridium ± 500 μm thick, the outermost layer (pellis) ± 150 μm thick with crowded flattened warts 55–180 μm tall and 100–375 μm broad, of ellipsoid to subglobose or subpolyhedral cells 6–30(−38) μm broad, those of the outer ± 50 μm of the ascomata surfaces with yellow to yellowish brown walls 1–3 μm thick imparting an orange brown color to the tissue, below which the inflated cells are hyaline and walls mostly < 1 μm thick; subpellis ± 350 μm thick, gradually to abruptly differentiated from the pellis as interwoven, hyaline, thin-walled hyphae 2–4(−8) μm broad. Asci hyaline, globose to ellipsoid or pyriform, 65–80 × 40–60 μm excluding the basal hyphal stem, 10–38 × 7–9 μm; walls thin (< 1 μm) in youth, with age thickened to 2 μm. Spores 1–5/ascus, ellipsoid to broadly ellipsoid, ornamented with crowded, discrete hyaline spines 3–4 × 1–1.5 μm. Spores in 1-spored asci (infrequent) 30–40 × 24.5–35 μm (Q = 1.18–1.45), 2-spored 25–33.5 × 21–28 μm (Q = 1.04–1.45), 3-spored 23–30 × 18.5–23 μm (Q = 1.21–1.32), 4-spored 20–26 × 14–21 μm (Q=1.19–1.4), 5-spored (infrequent) 18–26 × 15–21 μm (Q = 1.2–1.31); walls ± 2 μm thick, light yellow brown.
Distribution, habitat, and phenology: Cascade Mountains of far southwest Oregon and north on its western slopes as well as at the eastern slope of the Coast Ranges, thence to Washington’s far north in the San Juan Islands; elevations range from 20 to 1 545 m. Habitats range from various mixtures and ages of Pseudotsuga menziesii, Abies grandis, and Alnus rubra to in one case, a partially harvested old-growth and in another, a pure stand of Tsuga heterophylla. The one collection in August was relatively immature, the other three were well matured.
Paratypes: USA: Oregon, Benton County, Rock Creek Park (abandoned) off State Hwy. 34, elev. 137 m, in soil, 24 Aug. 1962, R. Benjamin No. 38, OSC 158256; Clackamas County, Hwy. 224, SW of Timber Lake, elev. 550 m, in soil, 1 Nov. 1995, A. Beyerle B316, OSC 148706 (Trappe 17457); Jackson Co., Conde Creek, elev. 1 545 m, in soil, 4 Nov. 2012, R. Brock RB12-139, OSC 151373.
DISCUSSION
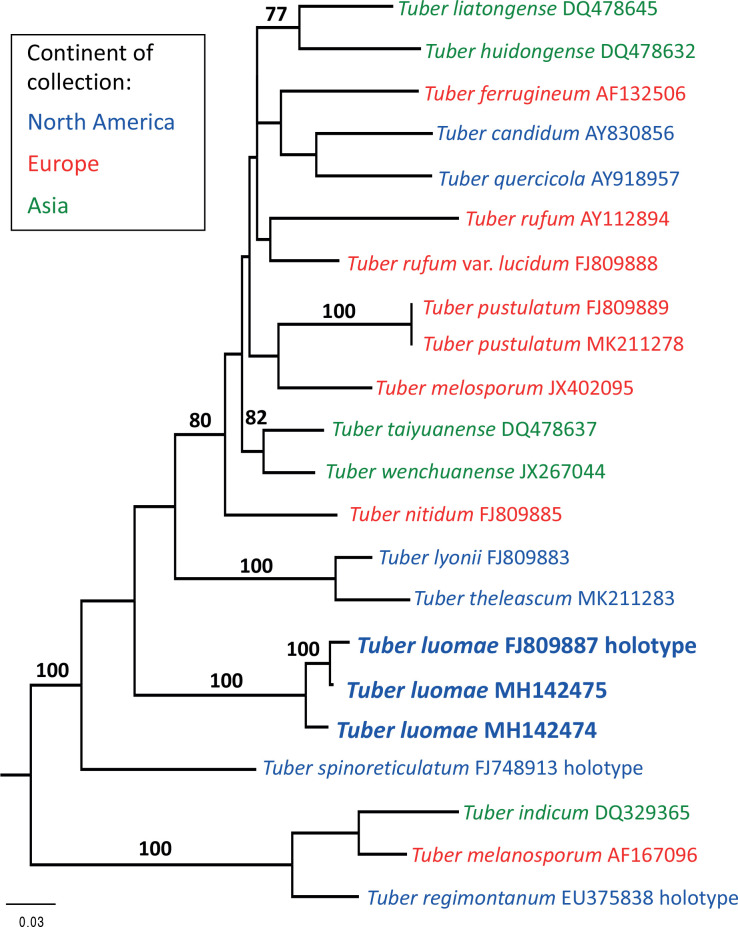
It is now evident from recently published molecular studies on Tuber and new species descriptions that Tuber is even more diverse than previously suspected (Bonito et al. 2010, Fan et al. 2011, Fan et al. 2012a, b, 2013, Alvarado et al. 2012a, Guevara et al. 2013, Zambonelli et al. 2016). In their assessment of the molecular diversity of Tuber, Bonito et al. (2010) and Healy et al. (2016) reported that the Rufum clade is among the more diverse in the genus and includes numerous cryptic undescribed species.
The Rufum clade is distributed across the Northern Hemisphere with centers of endemism in Europe, Asia, and North America (Bonito et al. 2013). While most species in this clade are characterized by spiny spores, some, such as T. spinoreticulatum and T. lyonii, have spiny spores with low reticulations (Uecker & Burdsall 1977, Trappe et al. 1996), whereas T. liaotongense has reticulate spores (Cao et al. 2011), and T. melosporum has smooth spores (Alvarado et al. 2012b).
Tuber luomae, known only from four collections in the Pacific Northwest (USA), is characterized by spiny-spores and a thick peridiopellis consisting of a pseudoparenchyma of inflated, subglobose to subpolygonal cells. Until recently, the only other described species in clade Rufum with a strongly pseudoparenchymatous pellis was T. malacodermum (Fischer 1923), known only from the holotype from Switzerland. Unlike T. luomae, it has a smooth peridiopellis, and the spines on its spores are occasionally to frequently connected by low ridges (‘lines’) on the spore surface, sometimes forming a partial reticulum. In addition, T. malacodermum has smaller asci but longer ascus stems than T. luomae. Despite their similar and distinctive peridial anatomy, they are readily distinguished by their spore ornamentation, ascus size, and ascus stem length.
Leonardi et al. (2019) examined the T. malacodermum holotype, as well as putative T. malacodermum ascomata collected in Spain, Corsica, and Mexico. Because morphological characters separated the type specimen from all the other collections, the authors concluded that the holotype is the only known collection of T. malacodermum. The type collection was too poorly preserved to yield DNA sequences, but molecular analysis of the newer species placed them into two previously unidentified groups. The specimens from Spain and Corsica were named T. pustulatum, whereas the Mexican specimens were named T. theleascum (Leonardi et al. 2019). Molecular analyses by Leonardi et al. (2019) show that T. luomae is more closely related to the North American T. theleascum than to the European T. pustulatum (Fig. 2).
Fig. 2.
Phylogenetic reconstruction of the Tuber rufum clade based on maximum likelihood analysis of the ITS rDNA region with 16 ingroup and 3 outgroup taxa (T. melanosporum, T. indicum, and T. regimontanum). The analysis was based on a GTR model of nucleotide substitutions and resulted in a most likely tree with a log likelihood score of –ln 4198.629. Scale bar = 0.03 substitutions per site.
Tuber luomae resembles T. pustulatum in the structure of the peridium; both species have a two-layered peridium with an outermost layer composed of globose to angular cells and a subpellis of interwoven hyphae. They differ in the spore ornamentation; spines are shorter in T. luomae (up to 4 μm long) in comparison with T. pustulatum (up to 7 μm long). Moreover, the spines in T. pustulatum are united at the base, forming a reticulum, while the spines in T. luomae are discrete (Fig. 1F, G). Tuber luomae is also similar to T. theleascum in peridial structure, but differs in the thickness of the peridium (T. theleascum 160–250 μm, T. luomae ± 500 μm). They also differ in spore ornamentation, with spines often interconnected in T. theleascum but not connected in T. luomae.
These four species (Tuber luomae, T. malacodermum, T. pustulatum and T. theleascum), which have in common a peridiopellis of isodiametric cells rather than the narrow hyphae of all other known members of the clade, are derived from separate lineages. We find it interesting that this peridial structure that is uncommon in the Rufum clade has been found in geographically disjunct species (Fig. 1). Tuber luomae has phylogenetic affinity to other North American Tuber species in the Rufum clade. In the Pacific Northwest, other Rufum clade species (T. candidum and T. quercicola) are readily distinguished from T. luomae by having a peridiopellis of tightly interwoven hyphae that lack or have only widely scattered inflated cells. Tuber luomae can be regarded as rare, considering that hypogeous sporocarps (truffles) have been extensively sought by many dozens of collectors producing thousands of collections over more than a century of searching in western Washington and Oregon.
ACKNOWLEDGEMENTS
The authors thank Dan Luoma, Richard Benjamin, Adrian Beyerle, and Richard Brock for their collections of T. luomae. Microslides were sectioned and stained by Darr M. Duff. JT and GB were supported in this work through National Science Foundation awards 0641297 and 1946445. We thank Oregon State University for accessioning the collections of Tuber luomae discussed in this paper, Kyle Gervers for assistance in the OSC herbarium during times of limited access, and MycoBank for registering the new name.
REFERENCES
- Alvarado P, Moreno G, Manjón LJ. (2012a). Comparison between Tuber gennadii and T. oligospermum lineages reveals the existence of the new species T. cistophilum (Tuberaceae, Pezizales). Mycologia 104: 894–910. [DOI] [PubMed] [Google Scholar]
- Alvarado P, Moreno G, Manjón LJ. (2012b). A new Tuber without spore ornamentation, Tuber melosporum comb. nov. Boletín de la Sociedad Micologia Madrid 36: 23–28. [Google Scholar]
- Bertini L, Amicucci A, Agostini D, et al. , (1999). A new pair of primers designed for amplification of the ITS region in Tuber species. FEMS Microbiology Letters 173: 239–245. [DOI] [PubMed] [Google Scholar]
- Bonito G, Smith ME, Nowak M, et al. , (2013). Historical biogeography and diversification of truffles in the Tuberaceae and their newly identified southern hemisphere sister lineage. PLOS one 8: 1–15, e52765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonito G, Gryganskyi AP, Trappe JM, et al. , (2010). A global meta-analysis of Tuber ITS rDNA sequences: species diversity, host associations and long-distance dispersal. Molecular Ecology 19: 4994–5008. [DOI] [PubMed] [Google Scholar]
- Cao JZ, Chi WD, Fan L, et al. , (2011). Notes on the neotype of Tuber taiyuanense. Mycotaxon 116: 7–11. [Google Scholar]
- Fan L, Cao JZ, Li Y. (2013). A reassessment of excavated Tuber species from China based on morphology and ITS rDNA sequence data. Mycotaxon 124: 155–163. [Google Scholar]
- Fan L, Cao JZ, Zheng ZH, et al. , (2012a). Tuber in China: T. microspermum and T. microspiculatum spp. nov. Mycotaxon 119: 391–395. [Google Scholar]
- Fan L, Hou CL, Cao JZ. (2011). Tuber sinoalbidum and T. polyspermum - new species from China. Mycotaxon 118: 403–410. [Google Scholar]
- Fan L, Hou CL, Li Y. (2012b). Tuber microverrucosum and T. huizeanum - two new species from China with reticulate ascospores. Mycotaxon 122: 161–169. [Google Scholar]
- Fischer E. (1923). Zur Systematik der schweizerischen Trüffeln aus den Gruppen von Tuber excavatum und rufum. Verhandlungen der Naturforschenden Gesellschaft in Basel 35: 34–50. [Google Scholar]
- Gardes M, Bruns TD. (1993). ITS Primers with enhanced specificity for Basidiomycetes - Application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Guevara G, Bonito G, Trappe JM, et al. , (2013). New North American truffles (Tuber spp.) and their ectomycorrhizal associations. Mycologia 105: 194–209. [DOI] [PubMed] [Google Scholar]
- Healy RA, Bonito G, Guevara G. (2009). The truffle genus Pachyphloeus in the U.S. and Mexico: phylogenetic analysis and a new species. Mycotaxon 107: 61–71. [Google Scholar]
- Healy RA, Bonito GM, Smith ME. (2016). A brief overview of systematics, taxonomy and ecology of the Tuber rufum clade. In: True truffle (Tuber spp.) in the world (Zambonelli A, Iotti M, Murat C, eds). Cham, Switzerland: Springer International Publishing. Soil Biology 47: 125–136. [Google Scholar]
- Johansen DA. (1940). Plantmicrotechnique. McGraw-Hill Book Co., Inc., NewYork. [Google Scholar]
- Leonardi M, Paz-Conde A, Guevara G, et al. , (2019). Two new species of Tuber previously reported as Tuber malacodermum. Mycologia 111: 376–389. [DOI] [PubMed] [Google Scholar]
- Maddison D, Maddison W. (2002). MacClade: Analysis of Phylogeny and Character Evolution. Version 4.0. Sunderland: Sinauer Associates. [Google Scholar]
- Trappe JM, Jumpponen A, Cázares E. (1996). NATS truffle and truffle-like fungi 5: Tuber lyonii (= T. texense), with a key to the spiny-spored species groups. Mycotaxon 60: 365–372. [Google Scholar]
- Uecker FA, Burdsall HH. (1977). Tuber spinoreticulatum, a new truffle from Maryland. Mycologia 69: 626–630. [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several species of Cryptococcus. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber NS, Trappe JM, Denison WC. (1997). Studies on western American Pezizales. Collecting and describing ascomata: macroscopic features. Mycotaxon 61: 153–176. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: A guide to Methods and Applications (Innis MA, Gelfand DH, Shinsky JJ, et al., , eds.), Academic Press, New York, USA: 315–322. [Google Scholar]
- Zambonelli A, Iotti M, Murat C, eds. (2016). True truffle (Tuber spp.) in the world. Cham, Switzerland: Springer International Publishing; Soil Biology 47. [Google Scholar]