Graphical abstract

Keywords: Quercetin, Gallic acid, Antioxidant, Kidney, Oxidative stress, HEK- 293 cells
Highlights
-
•
Gallic acid has better antioxidant protective effect than quercetin in vivo.
-
•
Quercetin has better antioxidant protective effect than gallic acid in vitro.
-
•
The antioxidant effect of quercetin was at the least concentration tested.
-
•
The antioxidant effect of gallic acid was at a higher concentrated tested.
-
•
The in vivo dosage for the antioxidant effects of quercetin in the kidney is low.
Abstract
Quercetin and gallic acid are phytochemicals with interesting pharmacological properties. We herein investigated the protective effect of quercetin (QUE) in comparison with gallic acid (GAL) against exogenously-induced oxidative damage in rats’ kidney and human embryonic kidney (HEK-293) cell lines. Adult Wistar rats were treated with QUE and GAL (50 mg/kg) separately or in combination with di-n-butylphthalate (DnBP) for 14 days; and HEK-293 cells were treated with different concentrations of GAL (25−294 μM) or QUE (2−17 μM or 28−165.43 μM) singly or in combination with H2O2 (200 μM). After treatment, the kidney and cell extracts were processed for biochemical analysis and histopathology. We found that GAL but not QUE prevented DnBP-induced increase in lipid peroxidation (2.603 ± 0.25 vs. 3.65 ± 0.21 μmol/mL). Treatment with QUE but not GAL was associated with increased plasma creatinine (729.09 ± 55.68 vs. 344.25 ± 50.78 μmol/l) and tissue malondialdehyde (3.72 ± 0.62 vs. 1.67 ± 0.47 μmol/mL) concentrations, along with histo-pathological changes such as glomerular and tubular degenerations. However, QUE exhibited wider therapeutic concentration ranges than GAL at which it inhibits lipid peroxidation in HEK-293 cells, and was found to inhibit H2O2-induced lipid peroxidation even at the lowest concentration (2 μM) that was tested (0.607 ± 0.074 vs. 0.927 ± 0.106 μmol/l). These suggest that the in vivo dosages required for the antioxidant protective effects of QUE in renal tissues are low.
1. Introduction
Phytochemicals found in fruits and vegetables are known to have antioxidant effects against pro-oxidative damage that is induced by environmental chemicals [1]. However, their protective effects against chemically-induced tissues damages are dependent on several factors such as dosages, molecular polarities, and experimental designs [[2], [3], [4]]. The optimal dosages of some phytochemicals required to provide protective effects against experimentally-induced tissue oxidative damages are often difficult to extrapolate. This is reported to be due to their low intestinal absorption in the biological system [5] or that different interstitial concentrations are required for different tissues [3]. For instance, gallic acid (GAL) has the capacity to act as pro-oxidant at high doses and produces deleterious effects in tissues [[6], [7], [8]] whereas, at low doses it acts as antioxidant and exerts beneficial effects on the health of humans including cardiovascular diseases, chemoprevention of cancers and mitochondrial damage of several tissues [[9], [10], [11]]. Furthermore, several studies with many phytochemicals including quercetin (QUE), hesperetin, naringenin, myricetin and morin have established that these flavonoids have both antioxidant and pro-oxidant effects at different doses [12,13]. The health benefits of GAL and QUE facilitate them to be used as antioxidant ingredients in poly (vinyl alcohol) film formulations for food packaging [14].
The tissues of animals are rich in antioxidants and radical scavengers such as glutathione (GSH), catalase (CAT) and superoxide dismutase (SOD) which protect them from oxidative stress and lipid peroxidative damage [4]. Tissues that are endowed with antioxidant protective agents are less vulnerable to oxidative damage and are said to be in good health status [4]. In our previous studies, GAL at 100 mg/kg body weight produces deleterious effects on the testis, kidney and liver [4,8].
In several in vivo studies with rats were GAL exhibits antioxidant effects, the doses used are lower than 100. Similarly, our previous studies with rodent models showed that low dosages of QUE (5−20 mg/kg body weight) produce beneficial effects on the antioxidant defence of several tissues [3] and exhibit anticancer and apoptosis-inducing effects in vitro and in vivo at higher doses [16] but not when oxidative stress had already been induced from exogenous sources [3]. For example, in atrazine-induced oxidative damage, QUE at 20 mg/kg body weight exacerbated the pro-oxidant effects of atrazine [2] whereas doses of QUE as low as 5 mg/kg body weight did not show protective effects in these experimental models [3]. The dose of QUE was required to be increased to 10 mg/kg body weight to provide protective effects against oxidative damage in the liver and kidney but not the brain tissue of adult rats [3]. Furthermore, doses of QUE higher than was used in the studies above [2,3] were also observed to have antioxidant protective effects in several tissues of different experimental models [17,18]. However, the antioxidant protective effects of QUE in tissues and cells at low doses were little in tissues/cells in the absence of exogenous stress [19]. Thus there appear to be difficulty in extrapolating a single dose for QUE that provide protective effects in tissues were oxidative stress was induced by environmental chemicals under different experimental settings. To further illustrate this, we studied the effect of QUE at a dose of 50 mg/kg body weight, and then compared its effects with GAL against exogenously-induced oxidative damage. Quercetin metabolites have been found in the plasma of rats administered with this dose of QUE [20], and consistent with previous reports, in which QUE was absorbed from the small intestine [21].
Phthalate esters are ubiquitously present in several consumer products and are known to induce oxidative stress in experimental models [22,23]. This effect has been associated with tissue atrophy, impaired tissue antioxidant defence and nephrotoxicity in different experimental models [[24], [25], [26], [27], [28]]. The role of oxidative stress in butyl phthalate -induced toxicity was fuadministration of antioxidants prevented phthalate ester-induced tissue damage in rats [22].
The purpose of this study was to evaluate the protective effects of QUE and GAL against induced oxidative stress in vivo in rats?" kidney and in vitro in HEK-293 human embryonic kidney cell lines. At the same dose tested (50 mg/kg) for both compounds, we observed from the in vivo data that GAL protected the kidney against DnBP-induced oxidative stress but QUE did not. But data from the in vitro study allowed us to suggest that low concentration of QUE (2 μM) protected HEK-293 cells from oxidative stress-induced by H2O2.
2. Materials and methods
2.1. Chemicals and reagents
Gallic acid, quercetin, hydrogen peroxide and butyl-phthalate (purity, > 98 %) were purchased from Sigma-Aldrich Corp. (St. Louis, MO USA). All other reagents were of analytical grade and were purchased from the British Drug Houses (Poole, Dorset, UK).
2.2. Animal model, experimental design and sample collection
Adult male Wistar rats (180-225 g) were purchased from the animal colony of the Department of Biochemistry, University of Port Harcourt, and kept in well-ventilated plastic cages. They were allowed to acclimatize for one week before the start of the experiment. The animals were maintained in a room under 12-h light: dark cycles and fed with rat's pellets and water ad libitum. The experimental protocols including the handling of the animals were approved by the Institutional Research Ethics Committee at the University of Port Harcourt and were in adherence with the endorsement of the National Institute of Health Guidelines for Animal Care and Use of Laboratory Animals (National Institute of Health Publication Number, 85-23).
The animals were randomized into six groups of five rats each and were treated as below:
Group I: Control/vehicle treated (corn oil), 2 mL/kg body weight, oral gavage, every other day for 14 days.
Group II: Di-n-butyl phthalate, 1 mL/kg body weight, oral gavage, every other day for 14 days
Group III: Gallic acid (GAL), 50 mg/kg body weight, oral gavage, every day for 7 days and every other day for 14 days.
Group IV: Gallic acid (as in group III) + Di-n-butyl phthalate (as in group II)
Group V: Quercetin (QUE), 50 mg/kg body weight, oral gavage, every day for 7 days and every other day for 14 days.
Group VI: Quercetin (as in group V) + Di-n-butyl phthalate (as in group II)
At the end of the study, the animals were starved overnight, weighed and sacrificed by cervical dislocation followed by decapitation. Blood samples were collected in heparin-coated bottles, allowed to stand for 1 h at room temperature and centrifuged at 4000 revolutions per minute for 15 min to obtain plasma. The two kidneys were removed, pat-dried between two sheets of filter paper and weighed. A 10 % homogenate of the right kidney was obtained by homogenizing the tissue in ice-cold 0.1 M Tris-HCl buffer (pH 7.4) followed by centrifugation at 5000g, 4 °C for 15 min. The separated tissue supernatant was used to evaluate the oxidative stress markers: malondialdehyde (MDA), reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT). The left kidneys were fixed in 10 % buffered neutral formalin solution for 24 h, dehydrated in graded alcohol series and embedded in paraffin wax according to the routine procedure. The tissues were cut (5 μm-thick sections) with a rotary microtome and stained routinely with haematoxylin and eosin for microscopy.
2.3. Culture of Human embryonic kidney cell line HEK-293 and treatment of cells
HEK-293 cells were the kind gift from Prof. Aristobolo M. Silva, Laboratories of Molecular Pathogenesis, Department of Morphology, Federal University of Minas Gerais, Belo Horizonte, Brazil. The cells were grown in Dulbecco?"s modified Eagle?"s medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin (Invitrogen) and 10 % bovine calf serum (Invitrogen) at 37 °C in a humidified incubator with 5% CO2. Before the cells were used for the experiment, they were subjected to viability assay using Trypan Blue, and batches showing more than 95 % viability were used for the experiment. Thereafter, the cells were seeded in 6-wells plate (Nunc International, Rochester, USA) at a density of 2 x 105 cells per well, and were allowed to reach 90-100 % confluence in 48-72 h. After pre-treatment of the cells with GAL (25 or 49 μM) or QUE (2-17 μM or 28-165.43 μM) for 1 h followed by treatment with 200 μM H2O2 [[28], [29], [30], [31], [32], [33]] for 2, 6 or 12 h, the cells were scraped from the culture plates, centrifuged at 5000 x g for 10 min and washed with phosphate buffer (pH 7.4). The cell extracts were suspended in 10 mM phosphate buffer (pH 7.4), homogenized for 30 s and then centrifuged at 5000 × g for 10 min at 4 °C to eliminate cellular debris.
2.4. Dose selection of tested compounds for the in vivo and in vitro study
The dose and route of administration of DnBP were established from earlier published studies [22,34]. Quercetin metabolites were measured in the plasma of rats [20] that were treated with QUE at the same dose that was tested in the present study, and the protective effect of gallic acid against chemically-induced oxidative damage in rat tissues has been reported previously at the same dose that was tested in the present study [15]. Many other laboratories have also reported the bioavailability of the tested compounds in rats when administered via the oral route [21,[35], [36], [37], [38], [39]]. To select the concentrations of the tested flavonoids for the in vitro study, we assumed that if there is equal distribution of the flavonoids throughout the body, and that gastrointestinal (GIT) absorption was 100 %, and the rate of GIT absorption of QUE or GAL was the same as that of the excretion/metabolism of both chemicals, then 50 mg/kg body wt. GAL or QUE administered to rats per day should approximate 50 μg/mL in the kidney tissues. This amount is equivalent to 294 μM of GAL and 165μM of QUE. We therefore selected GAL concentrations as 294, 147, 49 and 25 μM and QUE as 165, 83 and 28 μM. After treatment of cells with GAL (25-294 μM) or QUE (28-165 μM) for 24 h, the viability of the cells were tested by the MTT assay as reported previously [40]. All tested concentrations of QUE (28-165 μM) did not affect the viability of cells after 24 h. GAL at the concentrations (49-294 μM) tested were toxic to the cells, and the 49 μM concentration decreased cell viability by about 50 % (data not shown). Therefore the 49 μM GAL concentration and the 25 μM GAL concentration which was not toxic to the cells were used in the further studies.
2.5. Determination of oxidative stress markers
The concentration of MDA in the kidney homogenates and extracts from the cells were determined as previously described [41]. The absorbance of the sample mixture was read at 532 nm, and the MDA level was calculated from a standard curve and expressed in μmol MDA per mL. The concentration of GSH was determined in the kidney homogenate using the colouring reagent, 5,5⬲- dithio-bis (2-nitrobenzoic acid) [42]. The absorbance of the samples was read at 412 nm in a spectrophotometer and the GSH concentration was calculated from a standard curve and expressed μg GSH per mL. The enzyme activity of SOD in the tissue homogenate was assayed by the method of Misra and Fridovich [43]. This assay was based on the inhibition of epinephrine (0.01 %) autoxidation at pH 10.2. One unit of SOD activity was described as the amount of SOD required to cause 50 % inhibition of the oxidation of adrenaline to adrenochrome per min. The absorbance was read at 480 nm in a spectrophotometer against blank containing all the components except the sample. The activity of CAT was estimated in the tissue homogenates and cell extracts as previously reported [44]. The specific activity of CAT was calculated using the molar extinction coefficient of H2O2 at 240 nm, 43.59 L/mol cm. One unit of catalase activity is the amount of protein that converts 1 mmol H2O2 per min. The protein concentration in the tissue samples was determined by the method of Lowry using bovine serum albumin as the standard [45].
2.6. Determination of renal function markers
The separated plasma samples were processed for the determination of urea, creatinine (CREA) and uric acid using Randox commercial kits (RANDOX Laboratories Ltd., Crumlin, United Kingdom) with an autoanalyzer (LabTech, RE 1201007) and following the manufacturer?"s protocols.
2.7. Statistical analysis
Statistical analysis was performed using ANOVA and Tukey?"s post Hoc Test. Student?"s t-test was done when only two pairs of data were analysed. Data were expressed as mean ± SD, and p values less than 0.05 were considered to indicate statistical significance. All data were analysed with GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA, USA).
3. Results
3.1. Effects of QUE and GAL on body and kidney weights
Animals were observed daily during the experiment, and were in good health till the end of study. To further determine the toxic effects that are associated with the various treatments, the body and kidney weights of the animals were measured. There were no significant changes in body weight between the treated and control groups at 14 days exposure period (Data not shown). Additionally, there were no statistically significant changes (p > 0.05) in the average kidney weights of the animals at the end of the study (Data not shown).
3.2. Effects of QUE and GAL on DnBP-induced oxidative stress in the kidney
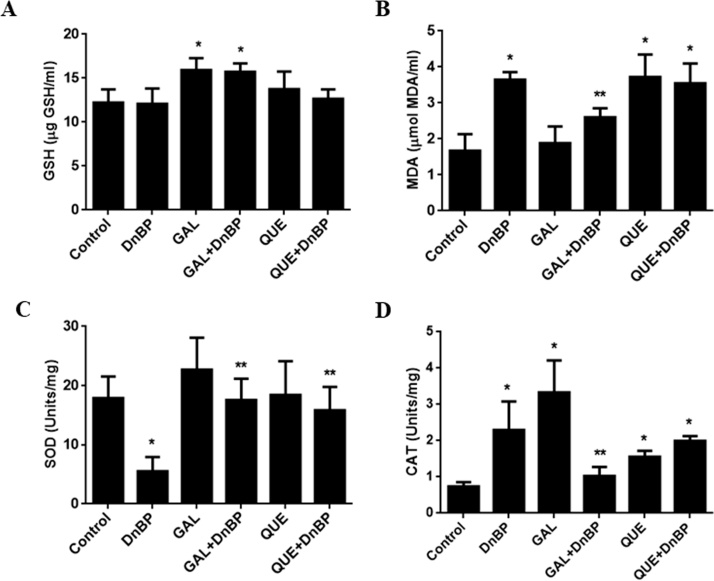
After 14 days of treatments with the tested chemicals, there were no significant changes found in GSH concentrations in the kidney of the QUE, DnBP and DnBP + QUE groups. The level of GSH in the kidney was significantly increased in the GAL and GAL + DnBP groups when compared with the control [Fig. 1A]. The level of MDA was significantly higher in the kidney of DnBP, QUE and QUE + DnBP groups when compared with the control. There were no significant changes in MDA concentrations in the kidneys of GAL animals when compared with the control group. The concentration of MDA in the kidney homogenates were significantly (p < 0.05) decreased in the GAL + DnBP group compared to DnBP treated animals [Fig. 1B]. The activity of SOD was significantly decreased in the kidney of rats treated with DnBP compared to the control group. There were no significant differences found on SOD activities in the kidney after GAL or QUE treatment. The activity of SOD was significantly higher in the GAL + DnBP group compared to the DnBP treated animals. In the QUE + DnBP treated animals, SOD activity was significantly increased when compared to the DnBP treated animals [Fig. 1C]. The activity of CAT was significantly higher in the kidney of DnBP-treated animals when compared with the control. The kidney homogenates of GAL or QUE treated animals also showed higher activity of CAT when compared with the animals in the control group. In the GAL + DnBP-treated animals, CAT activities were significantly lower when compared to the values obtained from the DnBP treated animals. There was no significant difference found in CAT activity in the kidneys of QUE + DnBP-treated animals compared to the DnBP treated animals [Fig. 1D].
Fig. 1.
Effects of quercetin (QUE) and gallic acid (GAL) on the level of (A) glutathione (GSH), (B) malondialdehyde (MDA), (C) superoxide dismutase (SOD), and (D) catalase (CAT) in the kidney, of rats after 14-days of oral treatment with di-n-butyl phthalate (DnBP). Values are mean ± SD; n = 5, *p < 0.05 versus control; **p < 0.05 versus DnBP.
3.3. Effects of QUE and GAL on H2O2-induced oxidative stress in HEK-293 cells
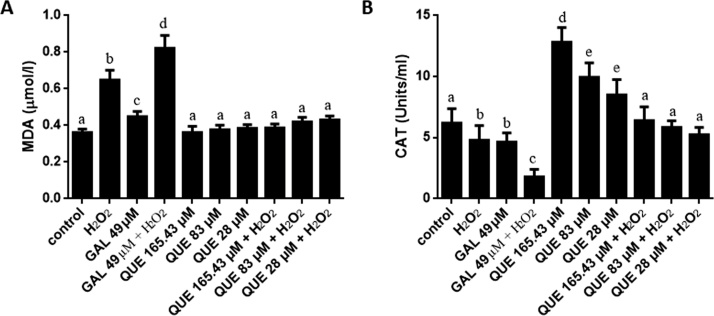
HEK-293 cells stimulated with H2O2 for 2 h led to an increase in CAT activity but did not change MDA level compared to the control [Fig. 2]. GAL (25 μM) treated cells did not show any change in MDA level, but CAT activity was increased in these cells. In the combined treatment groups, GAL (25 μM) prevented H2O2-induced increase in CAT activity. Treatment with QUE was observed to increase CAT activity up to the 331 μM concentration followed by a decrease onwards in a dose-dependent manner (415-1 mM). The increased CAT activity that was induced by H2O2 was decreased on co-treatment with QUE in a dose-dependent manner. As expected, QUE (165.43 μM) treatment alone or in combination with H2O2 did not change MDA values when compared to the control. Higher concentrations of QUE (331 μM - 1 mM) were found to increase MDA level in a dose-dependent manner when compared to control values, and these effects were synergistic on co-treatment with H2O2 [Fig. 2]. After 6 h of treatment, HEK-293 cells stimulated with H2O2 showed an increase in both CAT activity and MDA level which were decreased on co-treatment with GAL (25 μM). Cells treated with GAL (25 μM) alone did not change MDA level but increased CAT activity when compared with the control [Fig. 3]. Treatment of cells with lower concentrations of QUE (2-17 μM) did not alter MDA level and CAT activity when compared to the control but prevented H2O2-induced increased in CAT activity and MDA level [Fig. 3]. After treatment of HEK-293 cells with H2O2 for 12 h, CAT activity was decreased and MDA concentration was increased [Fig. 4]. Treatment of cells with GAL (49 μM) decreased CAT activity and increased MDA concentration. The decrease in CAT activity along with increase in MDA concentration were synergistic in the GAL 49 μM + H2O2 treated cells [Fig. 4]. Treatment of HEK-293 cells with QUE (28-165.43 μM) dose-dependently increased CAT activity, and have no effect on the concentration of MDA. In the combined treatment groups, QUE prevented H2O2-induced decrease in CAT activity and increase in MDA level in a dose-dependent fashion.
Fig. 2.
Effects of high concentrations of quercetin (QUE, 165.43 μM – 1 mM) in comparison with gallic acid (GAL, 25 μM) alone or in combination with hydrogen peroxide (H2O2) (200 μM) on (A) malondialdehyde (MDA) level and (B) catalase (CAT) in HEK-293 cells after 2 h. Experiment were performed three times and analysed in triplicates. The data were calculated by taking the mean of three independent experiments and at least three replicates were used in each experiment. *p < 0.05 versus control; **p < 0.05 versus hydrogen peroxide (H2O2).
Fig. 3.
Effect of low concentrations of quercetin (QUE, 2-17 μM) in comparison with gallic acid (GAL, 25 μM) alone and in combination with hydrogen peroxide (H2O2) (200 μM) on (A) malondialdehyde (MDA) level and (B) catalase (CAT) in HEK-293 (human embryonic kidney-293) cells after 6 h. The data were calculated by taking the mean of three independent experiments and at least three replicates were used in each experiment. *p < 0.05 versus control; **p < 0.05 versus hydrogen peroxide (H2O2).
Fig. 4.
Effects of different concentrations of quercetin (QUE, 28-165.43 μM) in comparison with gallic acid (GAL, 49 μM) against hydrogen peroxide (H2O2)-induced changes in (A) malondialdehyde (MDA) level and (B) catalase (CAT) activity in HEK-293 (human embryonic kidney-293) cells after 12 h. The data were calculated by taking the mean of three independent experiments and at least three replicates were used in each experiment. Bars with different letters are different from each other.
3.4. Effect of QUE and GAL on DnBP-induced changes in the plasma profiles of renal function markers
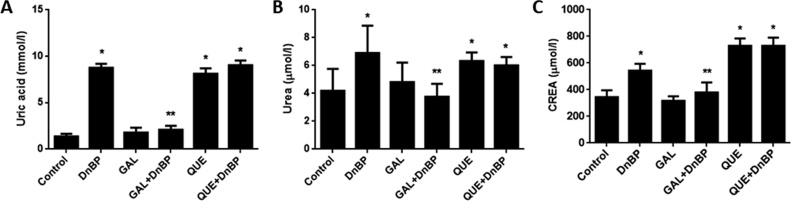
The plasma levels of uric acid, urea, and CREA were significantly higher in the DnBP-, QUE- and QUE + DnBP-treated rats when compared to the control values. There were no significant differences found in the levels of these renal markers when the values of the GAL-treated animals were compared to the control group. The plasma concentration of uric acid, urea, and CREA were significantly decreased in the GAL + DnBP treated animals when compared to the DnBP group [Fig. 5].
Fig. 5.
Effects of quercetin (QUE) and gallic acid (GAL) on the plasma indices of renal functions of rats after 14-days of oral treatment with di-n-butyl phthalate (DnBP). Values are mean ± SD; n = 5, *p < 0.05 versus control; **p < 0.05 versus DnBP. CREA = creatinine.
3.5. Effect of QUE and GAL on DnBP-induced histopathology of the kidney of rats
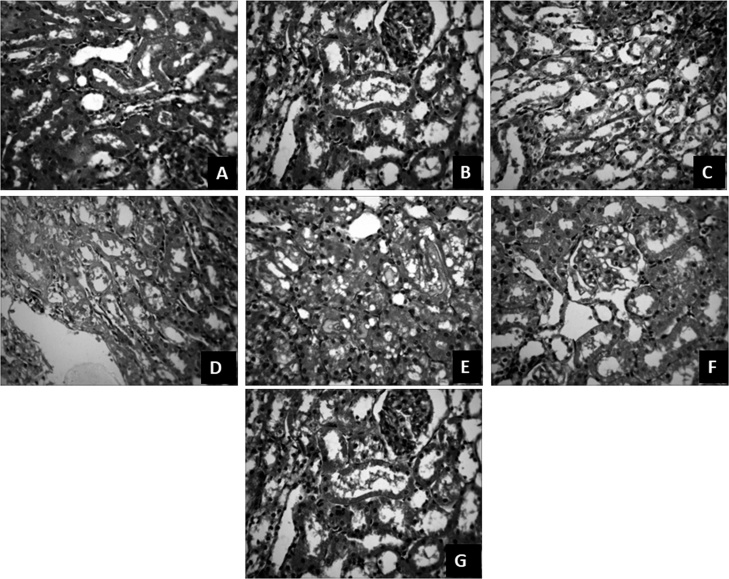
Histological examination of the kidney of the control and GAL groups appears normal with no visible lesion. In the kidney of the DnBP- treated animals, many of the tubules are degenerate and have protein casts in the lumina, and showed diffuse glomerular necrosis similar to the kidney of the QUE + DnBP treated animals. The kidney sections of the DnBP + GAL treated animals contain mild to moderate glomerular and tubular degeneration and necrosis. Histologic sections of the kidney of QUE treated animals also showed diffuse severe glomerular and tubular degeneration [Fig. 6].
Fig. 6.
Cross sections of the kidney of animals at the end of study. Control (A): No visible lesion seen; DnBP treated rats (B and C): many tubules are degenerate and contain protein casts in the lumina (B) and have diffuse severe glomerular and tubular degeneration and necrosis (C); GAL treated rats (D): No visible lesion seen; DnBP + GAL treated rats (E): mild to moderate glomerular and tubular degeneration and necrosis; QUE treated rats (F): diffuse severe glomerular and tubular degeneration; DnBP + QUE-treated rats (G): many tubules are degenerate and contain protein casts in the lumina. Mag ×400, H & E. di-n-butyl phthalate = DnBP; quercetin = QUE; gallic acid = GAL.
4. Discussion
There are several factors that interfere with the beneficial effects of phytochemicals in experimental animal models including their poor intestinal absorption and distribution in the tissues of biological system, experimental designs and dosages [3,46]. We reported previously that QUE at 5 mg/kg body weight fails to block experimentally-induced oxidative stress in the kidney of adult rats [3]. In the present study, we use a higher dose of QUE (50 mg/kg body weight) that was previously reported to be absorbed in rats after oral administration [37,38], and compared the effects with the same dose of GAL against DnBP-induced oxidative damage in the kidney of rats. We used this dose expecting to achieve antioxidant effects of both compounds under the same experimental condition. Surprisingly, we observed that GAL was protective but QUE was not protective against oxidative damage in vivo in the kidney.
The pro-oxidant effect of QUE in the present study was confirmed by the increased CAT activity MDA concentration in the kidney homogenates of the treated animals. Consequently, the increased CAT activity was insufficient to cope with the high level of the peroxide formed, thereby allowing oxidative stress to be induced. Previous reports from several laboratories have also confirmed that QUE can cause lipid peroxidation [47,48], and that the tissue MDA content is a function of the concentration of QUE [48]. Other laboratories have also reported that DnBP-induced oxidative damage was associated with changes in the antioxidant activities of several tissues [[49], [50], [51], [52]], thereby supporting our results. Because of the observed pro-oxidant effects of QUE, it was expected that the levels of the renal function markers (uric acid, urea and CREA) in the QUE + DnBP-treated animals be at least similar to the values of the DnBP-treated animals, and support the fact that QUE at the tested dose was toxic and did not protect the kidney against DnBP-induced kidney damage. In contrast, GAL co-treatment normalized renal function and prevented DnBP-induced oxidative damage, and was therefore beneficial than QUE in attenuating DnBP-induced oxidative damage. The antioxidant efficacy of GAL against different chemical toxicants induced oxidative stress has been well established in several experimental models. For instance, in rat liver mitochondria ex vivo model, GAL prevented bisphenol-induced oxidative damage by inhibiting lipid peroxidation and increasing the content of GSH [53]. The antioxidant neuro-protective effects of GAL against induced neuropathic pain were also reported in mice [54], thereby supporting our present data on the antioxidant protective effect of GAL in rat kidney stimulated with DnBP. Additionally, the increased GSH concentration in the kidney of GAL-treated animals allowed for the tolerance of reactive oxygen species, thereby making the tissues potentially resistance to oxidative insult [55]. This could elucidate the mechanisms by which GAL inhibits chemically-induced oxidative tissue injury.
To further evaluate the antioxidant effects of QUE and GAL against oxidative damage, we stimulated HEK-293 cells with H2O2 and measured MDA level and CAT activity, and then compared the effects of QUE with that of GAL. In many in vitro studies, H2O2 has been the chemical of choice to induce oxidative stress, and was therefore used to stimulate oxidant damage in HEK-293 cells as reported previously [[37], [38], [39], [40], [41]]. At the different concentrations of QUE (2–165 μM) tested, QUE reduced H2O2-induced MDA level and CAT activity suggesting a potent antioxidant activity of QUE at these physiological concentrations in vitro. Higher concentrations of QUE (331 μM - 1 mM) were found to have pro-oxidant effects and exerted higher effects on MDA level and CAT on co-treatment with H2O2. The concentration of GAL was required to be reduced to 25 μM to block the oxidative stress induced by H2O2. The antioxidant-pro-oxidant effects of GAL have been reported previously to be tightly controlled by their concentrations [4,7]. Because we assumed that the 50 mg/kg doses for both GAL and QUE administered in vivo could be distributed to the kidney tissues to at least 50 μg/mL, a dose of which the equivalent for GAL is 294 μM that was toxic to HEK-293 cells, and the 165 μM equivalent for QUE that exhibited potent antioxidant effect, allowed us to conclude that QUE was more efficient as an antioxidant than GAL in vitro but not in vivo. Furthermore, the antioxidant effect of QUE against H2O2-induced oxidative damage was also observed when QUE concentration was reduced to 2 μM, suggesting a wider concentration margin in vitro for QUE antioxidant effect compared to GAL. Previously, low concentration of QUE (5 μM) was reported to block oxidative damage in HEK-293 cells [56], and even potent antioxidant effects of QUE have been observed previously at 2 μM [48]. Furthermore, QUE (0.65 μmol/l) found in the human plasma after dietary intake of an onion rich meal was reported to be a potent antioxidant with the capacity to inhibit lipid peroxidation [57,58]. Therefore lower concentrations of QUE have potent antioxidant protective effects in HEK-293 cells, and the capacity of QUE to modulate exogenously-induced oxidative stress is due to the concentration achieved in target tissues in vivo. Thus, the oxidative damage in the kidney that was observed in the QUE treated animals in the present study suggested a switch from the classical antioxidant properties of QUE observed previously at 20 mg/kg body weight [59] to the pro-oxidant effect observed in the present study at 50 mg/kg body weight. However, this dose of QUE that failed to protect against phthalate ester-induced oxidative stress in the kidney were found, and even at higher doses, to exert antioxidant protective effects in oxidative stress models induced by other chemical agents e.g. aflatoxin [17] and imidacloprid [18]. In the study by Hassan et al. [18] were the nephro-protective effect of QUE was reported at a dose higher than was used in the present study, QUE was administered before the animals were challenged with the chemical toxicant for 21 days, whereas in the present study, QUE was administered daily for 7 days after which both tested chemicals were administered concurrently for 14 days. The co-treatment of the tested chemicals for a shorter duration and the less number of times the animals were co-treated with QUE might have influence our present results. Another possibility of the disparity in our results with these studies [17,18] could be that the interaction between butyl phthalate and QUE might have generated toxic metabolites in vivo that have potent pro-oxidant properties in the kidney [3,47]. Hence, the optimal dosages of QUE required to provide antioxidant protective effects in chemically-induced tissue oxidative damages are complex to extrapolate and cannot be generalised.
Overall, it appears that the physiological concentration required for QUE to exhibit antioxidant effect in the kidney is small. Therefore, the 50 mg/kg body weight of QUE administered orally to adult rats in the present study could have been distributed at a high amount in the target tissue. To support these claims, de Boer et al. [60] reported that QUE and its metabolites were increased in the kidney of rats after a long term dietary intake, and identified the kidney as one of the possible target tissues for QUE actions. In conclusion, GAL exhibits more versatility than QUE in protecting the kidney against oxidative damage in vivo whereas QUE has a wide concentration margin in vitro at which it exerts potent antioxidant effects. This new information is important in studies that aim to clarify the effective doses of QUE in clinical and non-clinical toxicology research.
Disclosure statement
The authors approve the submission of this manuscript and declare no conflict of interest.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
We gratefully acknowledge the generous gift of the HEK-293 cells from Professor Aristobolo M. Silva, Laboratories of Molecular Pathogenesis, Department of Morphology, University of Minas Gerais, Belo Horizonte, Brazil. We also thank Mr Kingsley Nwaugha for the collection of blood samples from the animals used in this study. The technical assistance of technologists in the Postgraduate Research Laboratories, Department of Biochemistry is gratefully acknowledged.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.07.015.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Surh Y.Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 2.Farombi E.O., Abarikwu S.O., Adesiyan A.C., Oyejola T.O. Quercetin exacerbates the effects of sub-acute treatment of atrazine on reproductive tissue antioxidant defence system, lipid peroxidation and sperm quality in rats. Andrologia. 2013;45:256–265. doi: 10.1111/and.12001. [DOI] [PubMed] [Google Scholar]
- 3.Abarikwu S.O. Protective effect of quercetin on atrazine induced oxidative stress in the liver, kidney, brain and heart of adult Wistar rats. Toxicol. Int. 2014;21:148–155. doi: 10.4103/0971-6580.139794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abarikwu S.O., Akiri O.F., Durojaiye M.A., Alabi A.F. Combined administration of curcumin and gallic acid inhibits gallic acid-induced suppression of steroidogenesis, sperm output, antioxidant defences and inflammatory responsive genes. J. Steroid Biochem. Mol. Biol. 2014;143:49–60. doi: 10.1016/j.jsbmb.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Tran T.H., Song Y.Guo D., Bruno R.S., Lu X. Quercetin-containing self-nano emulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci. 2014;103:840–852. doi: 10.1002/jps.23858. [DOI] [PubMed] [Google Scholar]
- 6.Niho N., Shibutani M., Tamura T., Toyoda K., Uneyama C., Takahashi N., Hirose M. Sub chronic toxicity study of gallic acid by oral administration in F344 rats. Food Chem. Toxicol. 2001;39(2001):1063–1070. doi: 10.1016/s0278-6915(01)00054-0. [DOI] [PubMed] [Google Scholar]
- 7.Park W., Chang M.S., Kim H., Choi H.Y., Yang W.M., Kim D.R., Park E.H., Park S.K. Cytotoxic effect of gallic acid on testicular cell lines with increasing H2O2 level in GC-1 spg cells. Toxicol. In Vitro. 2008;22:159–163. doi: 10.1016/j.tiv.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Abarikwu S.O., Durojaiye M., Alabi A., Asonye B., Akiri O. Curcumin protects against gallic acid-induced oxidative stress, suppression of glutathione antioxidant defences, and hepatic and renal damage in rats. Ren. Fail. 2015;38:321–329. doi: 10.3109/0886022X.2015.1127743. [DOI] [PubMed] [Google Scholar]
- 9.Choi E.J., Lee B.H., Lee K., Chee K.M. Long-term combined administration of quercetin and daidzein inhibits quercetin-induced suppression of glutathione antioxidant defences. Food Chem. Toxicol. 2005;43:793–798. doi: 10.1016/j.fct.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Prasad L., Khan T.H., Jahangir T., Sultana S. Effect of gallic acid on renal bio- chemical alterations in male Wistar rats induced by ferric nitriloacetic acid. Hum. Exp. Toxicol. 2006;25:523–529. doi: 10.1191/0960327106het652oa. [DOI] [PubMed] [Google Scholar]
- 11.Dutta M., Paul G. Gallic acid protects rat liver mitochondria ex vivo from bisphenol A induced oxidative stress mediated damages. Toxicol. Rep. 2019;6:578–589. doi: 10.1016/j.toxrep.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laughton M.J., Halliwell B., Evans P.J., Hoult J.R. Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricetin: effects of lipid peroxidation, hydroxyl radical generation and bleomycin dependent damage to DNA. Biochem. Pharmacol. 1989;38:2859–2864. doi: 10.1016/0006-2952(89)90442-5. [DOI] [PubMed] [Google Scholar]
- 13.Galati G., Moridani M.Y., Chan T.S., O’Brien P.J. Peroxidative metabolism of apigenin and naringenin versus luteolin and quercetin: glutathione oxidation and conjugation. Free Radic. Biol. Med. 2001;30:370–382. doi: 10.1016/s0891-5849(00)00481-0. [DOI] [PubMed] [Google Scholar]
- 14.Luzi F., Pannucci E., Santi L., Kenny J.M., Torre L., Bernini R., Puglia D. Gallic acid and quercetin as intelligent and active ingredients in poly(vinyl alcohol) films for food packaging. Polymers. 2019;11:1999. doi: 10.3390/polym11121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijaya Padma P., Sowmya T., Arun Felix R., Baskaran P., Poornima P. Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem. Toxicol. 2011;49:991–998. doi: 10.1016/j.fct.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Hashemzaei M., Delarami Far A., Yari A., Heravi R.E., Tabrizian K., Taghdisi S.M., Sadegh S.E., Tsarouhas K., Kouretas D., Tzanakakis G., Nikitovic D., Anisimov N.Y., Spandidos D.A., Tsatsakis A.M., Rezaee R. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017;38:819–828. doi: 10.3892/or.2017.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Nekeety A.A., Abdel-Azeim S.H., Hassan A.M., Hassan N.S., Aly S.E., Abdel-Wahhab M.A. Quercetin inhibits the cytotoxicity and oxidative stress in liver of rats fed aflatoxin-contaminated diet. Toxicol. Rep. 2014;1:319–329. doi: 10.1016/j.toxrep.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan A.M.S., Abo El-Ela F.I., Abdel-Aziz A.M. Investigating the potential protective effects of natural product quercetin against imidacloprid-induced biochemical toxicity and DNA damage in adults rats. Toxicol. Rep. 2019;6:727–735. doi: 10.1016/j.toxrep.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaindl U., Eyberg I., Rohr-Udilova N., Heinzle C., Marian B. The dietary antioxidants resveratrol and quercetin protect cells from exogenous pro-oxidative damage. Food Chem. Toxicol. 2008;46:1320–1326. doi: 10.1016/j.fct.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto T., Ueda Y., Oi N., Sakakibara H., Piao C., Ashida H., Goto M., Kanazawa K. Effect of combine administration of quercetin, rutin and extract of white radish sprout rich in kaempferol glycosides on the metabolism of rats. Biosci. Biotechnol. Biochem. 2006;70:279–281. doi: 10.1271/bbb.70.279. [DOI] [PubMed] [Google Scholar]
- 21.Hollman P.C.H., Bijsman M.N.C.P., van Gameren Y., Cnossen E.P., de Vries J.H.M., Katan M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999;31:569–573. doi: 10.1080/10715769900301141. [DOI] [PubMed] [Google Scholar]
- 22.Farombi E.O., Abarikwu S.O., Adedara I.A., Oyeyemi M.O. Curcumin and kolaviron ameliorate di-n-butyl phthalate-induced testicular damage in rats. Basic Clin. Pharmacol. Toxicol. 2007;100:43–48. doi: 10.1111/j.1742-7843.2007.00005.x. [DOI] [PubMed] [Google Scholar]
- 23.Cheng L., Li J., Cheng J., Wu Z. Dibutyl phthalate-induced activation of ROS and ERK1/2 causes hepatic and renal damage in Kunming mice. Hum. Exp. Toxicol. 2019;38:938–950. doi: 10.1177/0960327119843583. [DOI] [PubMed] [Google Scholar]
- 24.Wellejus A., Dalgaard M., Loft S. Oxidative DNA damage in male Wistar rats exposed to di-n-butyl phthalate. J Toxicol Environ Health A. 2002;65:813–824. doi: 10.1080/00984100290071126. [DOI] [PubMed] [Google Scholar]
- 25.Tseng I.-L., Yang Y.-F., Yu C.-W., Li W.H., Liao V.H.C. Phthalates induce neurotoxicity affecting locomotor and thermotactic behaviors and AFD neurons through oxidative stress in Caenorhabditis elegans. PLoS One. 2013;8:e82657. doi: 10.1371/journal.pone.0082657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wójtowicz A.K., Szychowski K.A., Wnuk A., Kajta M. Dibutyl Phthalate (DBP)-induced apoptosis and neurotoxicity are mediated via the aryl hydrocarbon receptor (AhR) but not by estrogen receptor alpha (ERα), estrogen receptor Beta (ERβ), or peroxisome proliferator-activated receptor gamma (PPARγ) in mouse cortical neurons. Neurotox. Res. 2017;31:77–89. doi: 10.1007/s12640-016-9665-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abd-Ellah M.F., Aly H.A.A., Mokhlis H.A.M., Abdel-Aziz A.H. Quercetin attenuates di-(2 ethylhexyl) phthalate-induced testicular toxicity in adult rats. Hum. Exp. Toxicol. 2016;35:232–243. doi: 10.1177/0960327115580602. [DOI] [PubMed] [Google Scholar]
- 28.Woodward K.N. Phthalate esters, cystic kidney disease in animals and possible effects on human health: a review. Hum. Exp. Toxicol. 1990;9:397–401. doi: 10.1177/096032719000900607. [DOI] [PubMed] [Google Scholar]
- 29.Zmijewski J.W., Banerjee S., Bae H., Friggeri A., Lazarowski E.R., Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J. Biol. Chem. 2010;285:33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto K., Kushima R., Kisaki O., Fujiyama Y., Okabe H. Combined effect of hydrogen peroxide induced oxidative stress and IL-1α on IL-8 production in Caco-2 cells (a human colon carcinoma cell line) and normal intestinal epithelial cells. Inflammation. 2003;27:123–128. doi: 10.1023/a:1023813710941. [DOI] [PubMed] [Google Scholar]
- 31.Wijeratne S.S., Cuppett S.L., Schlegel V. Hydrogen peroxide induced oxidative stress damage and antioxidant enzyme response in Caco-2 human colon cells. J. Agric. Food Chem. 2005;53:8768–8774. doi: 10.1021/jf0512003. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima Y., Nishida H., Nakamura Y., Konishi T. Prevention of hydrogen peroxide-induced oxidative stress in PC12 cells by 3,4-dihydroxybenzalacetone isolated from Chaga (Inonotus obliquus (person) Pilat) Free Radic. Biol. Med. 2009;47:1154–1161. doi: 10.1016/j.freeradbiomed.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 33.Hou X., Tong Q., Wang W., Xiong W., Shi C., Fang J. Dihydromyricetin protects endothelial cells from hydrogen peroxide-induced oxidative stress damage by regulating mitochondrial pathways. Life Sci. 2015;130:38–46. doi: 10.1016/j.lfs.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Gray T.J., Rowland I.R., Foster P.M., Gangolli S.D. Species differences in the testicular toxicity of phthalate esters. Toxicol. Lett. 1982;11:141–147. doi: 10.1016/0378-4274(82)90119-9. [DOI] [PubMed] [Google Scholar]
- 35.Zeng Q., Wei C., Wu Y., Li K., Ding S., Yuan J., Yang X., Chen M. Approach to distribution and accumulation of dibutyl phthalate in rats by immunoassay. Food Chem. Toxicol. 2013;56:18–27. doi: 10.1016/j.fct.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 36.Domingue-Romero E., Scheringer M. A review of phthalate pharmacokinetics in human and rat: what factors drive phthalate distribution and partitioning? J. Drug Metab. Rev. 2019;51:314–329. doi: 10.1080/03602532.2019.1620762. [DOI] [PubMed] [Google Scholar]
- 37.Manach C., Morand C., Demigne C., Texier O., Regerat F., Remesy C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997;409:12–16. doi: 10.1016/s0014-5793(97)00467-5. [DOI] [PubMed] [Google Scholar]
- 38.Kammalla A.K., Ramasamy M.K., Chintala J., Dubey G.P., Kaliappan I. Comparative pharmacokinetic interactions of quercetin and rutin in rats after oral administration of European patented formulation containing Hipphophae rhamnoides and co-administration of quercetin and rutin. Eur. J. Drug Metab. Pharmacokinet. 2015;40:277–284. doi: 10.1007/s13318-014-0206-9. [DOI] [PubMed] [Google Scholar]
- 39.Yu Z., Song F., Jin Y.-C., Zhang W.-M., Zhang Y., Liu E.-J., Zhou D., Bi L.-L., Yang Q., Li H., Zhang B.-L., Wang S.-W. Comparative pharmacokinetics of gallic acid after oral administration of gallic acid monohydrate in normal and isoproterenol-induced myocardial infarcted rats. Front. Pharmacol. 2018;9(2018):328. doi: 10.3389/fphar.2018.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abarikwu S.O., Pant A.B., Farombi E.O. 4-Hydroxynonenal induces mitochondrial mediated apoptosis and oxidative stress in SH-SY5Y human neuronal cells. Basic Clin. Pharmacol. Toxicol. 2012;110:441–448. doi: 10.1111/j.1742-7843.2011.00834.x. [DOI] [PubMed] [Google Scholar]
- 41.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 42.Sedlak J., Lindsay R.H. Estimation of total, protein bound, and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 43.Misra H.P., Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 44.Clairborne A. Catalase activity. In: Greewald A.R., editor. Handbook of Methods for Oxygen Radical Research. CRC Press Ltd; Boca Raton: 1995. pp. 237–242. [Google Scholar]
- 45.Lowry O.H., Rosenbrough N.J., Farr A.L., Randall R.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 46.Bouayed J., Bohn T. Exogenous antioxidants-double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010;3:228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metodiewa D., Jaiswal A.K., Cenas N., Dickancaité E., Segura-Aguilar J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic. Biol. Med. 1999;26:107–116. doi: 10.1016/s0891-5849(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 48.Filipe P., Haigle J., Silva J.N., Freitas J., Fernandes A., Mazière J.C. Anti- and pro-oxidant effects of quercetin in copper induced low density lipoprotein oxidation. Quercetin as an effective antioxidant against pro-oxidant effects of urate. Eur. J. Biochem. 2004;271:1991–1999. doi: 10.1111/j.1432-1033.2004.04111.x. [DOI] [PubMed] [Google Scholar]
- 49.Seo K.W., Kim K.B., Kim Y.J., Choi J.Y., Lee K.T., Choi K.S. Comparison of oxidative stress and changes of xenobiotic metabolizing enzymes induced by phthalates in rats. Food Chem. Toxicol. 2004;42:107–114. doi: 10.1016/j.fct.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Zhou D., Wang H., Zhang J. Di-n-butylphthalate (DBP) exposure induces oxidative stress in epididymis of adult rats. Toxicol. Ind. Health. 2011;27:65–71. doi: 10.1177/0748233710381895. [DOI] [PubMed] [Google Scholar]
- 51.Zuo H.X., Li J.Q., Han B., Ke C.J., Liu X.D., Zhang Y.C., Li L., Yang X. Di-(n-butyl)-phthalate-induced oxidative stress and depression-like behaviour in mice with or without ovalbumin immunization. Biomed. Environ. Sci. 2014;27:268–280. doi: 10.3967/bes2014.001. [DOI] [PubMed] [Google Scholar]
- 52.Ryu J.Y., Lee E., Kim H.J., Park H., Im Y.J., Kim J., Han S.Y., Kang I.H., Park K.L., Kim H.S. Alterations of di (n-butyl) phthalate-induced oxidative stress in the testis of hypothyroid rats. Toxicol. Environ. Chem. 2008;90:113–126. [Google Scholar]
- 53.Dutta M., Paul G. Gallic acid protects rat liver mitochondria ex vivo from bisphenol A induced oxidative stress mediated damages. Toxicol. Rep. 2019;6:578–589. doi: 10.1016/j.toxrep.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaur S., Muthuraman A. Ameliorative effect of gallic acid in paclitaxel-induced neuropathic pain in mice. Toxicol. Rep. 2019;6:505–513. doi: 10.1016/j.toxrep.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cipak L., Berceliova E., Paulikova H. Effects of flavonoids on glutathione and glutathione-related enzymes in cisplatin-treated L1210 leukemia cells. Neoplasma. 2003;50:443–446. [PubMed] [Google Scholar]
- 56.Ben Salem I., Prola A., Boussabbeh M., Guilbert A., Bacha H., Abid-Essefi S. Crocin and quercetin protect HCT116 and HEK293 cells from Zearalenone-induced apoptosis by reducing endoplasmic reticulum stress. Cell Stress Chaperones. 2015;20:927–938. doi: 10.1007/s12192-015-0613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hollman P.C.H., Gaag M.V.D., Mengelers M.J.B., VanTrijp J.M.P., DeVries J.H.M., Katan M.B. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic. Biol. Med. 1996;21:703–707. doi: 10.1016/0891-5849(96)00129-3. [DOI] [PubMed] [Google Scholar]
- 58.Vinson J.A., Dabbagh Y.A., Serry M.M., Jang J. Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart disease. J. Agricul. Food Chem. 1995;43:2800–2802. [Google Scholar]
- 59.Olayinka E.T., Ore A., Ola O.S., Adeyemo A.O. Protective effect of quercetin on melphalan-induced oxidative stress and impaired renal and hepatic functions in rat. Chemother. Res. Pract. 2014;2014 doi: 10.1155/2014/936526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boer V.Cde, Dihal A.A., van der Woude H., Arts I.C., Wolffram S., Alink G.M., Rietjens I.M., Keijer J., Hollman P.C. Tissue distribution of quercetin in rats and pigs. J. Nutr. 2005;135:1718–1725. doi: 10.1093/jn/135.7.1718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.