Graphical abstract
Keywords: Pseudomonas stutzeri, Exopolysaccharide, Alginate
Highlights
-
•
Alginate produced as an exopolysaccharide from the bacterium Pseudomonas stutzeri.
-
•
16S rRNA sequence is submitted for the species Pseudomonas stutzeri TN_AlgSyn.
-
•
Extraction of alginate is done using various media and characterization of synthesized alginate supports the sequence of G and M blocks.
-
•
Homogeneity of the alginate is achieved in bacterial sources with reasonable yield.
-
•
Cytotoxic studies and metal leaching properties proves to be potent source.
Abstract
Alginate is a group of water-soluble linear polysaccharides comprising of variable units of α-l-guluronic and β-d-mannuronic acid. The alginates are in high demand in biomedical, pharmaceutical and bioengineering applications. In the present study, we have isolated a strain of Pseudomonas stutzeri that has potential alginate synthesis. The biochemical and physiochemical characteristic including Carbazole assay, DSC, FTIR and H NMR were confirmed the alginate synthesis efficacy by P. stutzeri. Evaluation of P. stutzeri alginate for the removal of heavy metals such as Chromium, Cobalt and Lead showed that it effectively adsorbs heavy metals. Further analysis of gelling ability and cytotoxicity evaluation revealed that the alginate can be reconstituted as hydrogel and scaffold. Overall, our findings suggest that the strain P. stutzeri TN_Alg Syn may be used to produce alginate at commercial level that has the potential bioremediation and biomedical applications.
1. Introduction
Alginate or alginic acid is a polysaccharide produced by various genera of marine brown algae and some bacteria such as Azotobacter and Pseudomonas [[1], [2], [3], [4], [5]]. Alginate consists of β-d-mannuronic acid (M) and α-l-guluronic acid (G) monomers joined together by 1,4-linkages, and are arranged in homogenous (poly-G, poly-M) or heterogenous (MG) block-like patterns [6]. The structural, chemical and functional properties of alginates vary depending on their source [7]. Alginate has gained a preferential place in several industrial applications including the heavy metal absorption from industrial waste, development of drug delivery systems and wound healing biomaterials because of its specialized properties such as swelling capacity, viscosifying property and lack of toxicity [5,8,9]. Due to its broad spectrum of biomedical applications, the demand for alginate has increased over time, and it is likely to increase significantly in the future [3,10]. Commercially, marine macroalgae or brown algae are used produce alginates worldwide [11]. As the natural resources for these alginophytes are limited and method like kelp farming have been developed for the cultivation of alginophytes in many countries. The kelp farming methodologies include the production of seedlings on strings (laboratory-culture stage) and their subsequent attachment to culture ropes for growth in a floating raft culture (sea-culture stage). The environmental and seasonal variations such as temperature, changes in water current etc. drastically affect the kelp farming as well as the homogeneity of the production quality [4,10]. In order to overcome these variations, and to accomplish the current and future demands of alginate, an alternative source for mass alginate production is needed.
Bacterial alginate could be a better alternative for brown algae-derived alginate because of its well-defined chemical structures and physical properties. Also, the production of alginate by bacteria offers several additional advantages, such as overcoming the seasonal variations and batch to batch variations in their production and the product homogeneity. The yield of alginate can also be controlled by altering the culture conditions as well as expected quality can be obtained by genetically modified microorganisms [12]. Bacterial alginate biosynthesis pathway has the following steps, (i) precursor substrate synthesis (ii) polymerization (iii) cytoplasmic and periplasmic membrane transport (iv) modification and (v) export through the outer membrane and secretion [13]. Several studies have reported that some bacterial strains including Pseudomonas species produce alginate as extracellular polysaccharides, in which majority of them lack the G-blocks [12]. The present study was initiated with screening of soil samples for bacteria which produce biopolymers, where we found a new strain of Pseudomonas stutzeri that have the capability of producing alginate. Further characterization of the purified alginates showed its efficacy towards bioremediation and biomedical applications are proposed.
2. Materials and methods
2.1. Isolation of exopolysaccharide producing bacteria
The bacteria were isolated from the rhizosphere of leguminous plant field in Madurai, India using routine microbiological techniques [14]. The isolated bacteria were maintained at 4 °C on nutrient agar slant. Ten individual isolates were screened for exopolysaccharide (EPS) synthesis. The characterization of isolated alginate producing bacteria was done by morphological, biochemical tests and genotypic methods as outlined below.
2.2. Molecular level characterization of isolate
The phylogenetic status of the isolate was determined by genotyping the gene encoding 16S rRNA. In brief, the bacterial genomic DNA was isolated using the InstaGeneTM Matrix Genomic DNA isolation kit (cat # 732-6030 Bio-Rad Laboratories Pvt Ltd), and the 16S rRNA gene fragment was amplified by polymerase chain reaction (PCR) with a forward primer (Sense - 5′-AGAGTTTGATCMTGGCTCAG-3′) and a reverse primer (Antisense – 5′-TACGGYTACCTTGTTACGACTT-3′) using MJ Research Peltier Thermal Cycler. The PCR product was purified with Montage PCR Clean up kit (cat # P36322, Millipore) and the DNA sequence was determined using Illumina hiseq sequencer (YAAZH XENOMICS, India). The 16S rRNA sequence was analyzed by using NCBI BLAST similarity search tool and the multiple sequence alignment (Phylogeny analysis) was performed with the closely related sequence of blast results. The program MUSCLE 3.7 was used for multiple alignments of sequences and the resulting aligned sequences were cured using the program Gblocks 0.91b (to remove alignment noise) [15]. The phylogeny analysis was done with PhyML 3.0 aLRT and HKY85 was used as a substitution model [16].
2.3. Evaluation of EPS synthesis by Pseudomonas stutzeri
Pseudomonas stutzeri growth and the potential for EPS synthesis was tested in two different growth media, basal medium I and II and compared for the production of bacterial EPS according to previous protocol with slight modification [17]. Specifically, the basal medium I comprised MOPS and FeSO4, whereas basal medium II was without MOPS and replaced FeSO4 with FeCl3. The media compositions are as follows; The basal medium I (g/L): Yeast extract- 3; K2HPO4- 0.66; KH2PO4- 0.16; MgSO4.7H2O- 0.2; CaSO4.2H2O- 0.05; Na2MoO4.2H2O- 0.0029; FeSO4.7H2O- 0.002; MOPS- 1.42; Sucrose- 20, pH 7.0. The basal medium II (g/L): Yeast extract, 3; K2HPO4- 0.8; KH2PO4- 0.2; MgSO4.7H2O- 0.2; CaSO4.2H2O- 0.1; Na2MoO4.2H2O- 0.001; FeCl3- 0.027; Sucrose- 20, at pH 7.0. Pseudomonas stutzeri was cultured in 500 ml culture flask at 30 °C for 12–25 days with constant shaking of 200 rpm. Each experiment was done in triplicate. Triplicate cultures were used for each batch experiment.
2.4. Batch culture
The batch culture was conducted using protocol described earlier with some modifications [18]. In brief, the seed culture was prepared in a 500 ml Erlenmeyer flask containing 150 ml growth media using Pseudomonas stutzeri. Batch cultivation was carried out at optimum temperature (30 °C) in a 7.5 L bioreactor (BioFlo/Celligen 115, New Brunswick, USA) containing 3 L of media. The bioreactor was sterilized at 121 °C for 20 min, cooled and then inoculated with 50 ml inoculum. The pH of the culture broth was maintained at 7.0 by automatic addition of acid or base by pH–mV controller (MettlerTolledo, USA). Dissolved oxygen was measured by DO probe (MettlerTolledo, USA) and the concentration was maintained at 30 % saturation value by cascading the speed of the agitator and air flow rate. The initial substrate concentration (sucrose) was 20 g/l and fermentation was carried out for 25 days. The growth was monitored at regular time intervals and the dry cell mass was estimated.
2.5. Collection of bacterial exopolysaccharide
After minimum of 14 days of incubation, semisolid beads of EPS production was observed. The EPS beads were collected, washed with aqueous solution followed by ethanol (95 %) and stored in the refrigerator at 4 °C. It is then dried in hot air oven at 50−60 °C and used for further analysis.
2.6. Confirmation of alginate synthesized by Pseudomonas stutzeri
2.6.1. Determination of carbohydrates
To determine the nature of carbohydrate present in the harvested exopolysaccharide, Molisch’s test, Iodine test, Seliwanoff test and Osazone test were done according to standard procedures [19].
2.6.2. Determination of uronic acid
The presence of uronic acid was determined by using carbazole method [20]. In brief, a 50 μl of serially diluted standard alginic acid and synthesized bacterial alginate (100 mg/ml) were placed in a 96-well plate and 200 μl of a solution of 25 mM sodium tetra borate (in sulfuric acid) was added. The plate was heated at 100 °C for 10 min and cooled for 15 min at room temperature. Then, 50 μl of 0.125 % carbazole (in ethanol) was added, heated again at 100 °C for 10 min and cooled at room temperature for 15 min. The absorption was measured at 550 nm in micro plate reader.
2.6.3. Fourier transform infrared spectroscopy (FTIR spectroscopy)
The chemical structure of monomers present in the bacterial alginate was determined by FT-IR. In brief, spectra of harvested bacterial alginate samples were recorded with IRPrestige-21 FTIR spectrophotometer, (Shimadzu) in a spectra ranging from 4000 cm−1 to 400 cm−1 at 4 cm−1.
2.6.4. 1H Nuclear magnetic resonance (NMR)
The proton and carbon number of harvested alginate were identified and confirmed by 1H NMR experiments using a Bruker AVANCE HD 500 MHz FT-NMR Spectrometer (IITM, Chennai-TN, India). In brief, 5 mg of bacterial alginate was dissolved in 1 ml of D2O (Deutrium dioxide) and the spectra were recorded at 400.13 MHz, 100.62 frequencies, an 90° pulse, and an acquisition time of 2.5 s. using 1.7 mm Triple Inverse Probe with gradient (TXI) broadband probe head equipped with shielded z-gradient. The spectra were analyzed with Windows 7 based TOPSPIN 3.5 software.
2.6.5. Differential scanning calorimetry (DSC)
DSC thermograms of vacuum-dried bacterial alginate beads were analyzed using NETTZSCH DSC 204 (SAIF laboratory, IITM, Chennai-TN). Briefly, 15 mg of the sample was placed in aluminium pan and heated from 25 °C to 400 °C at a scanning rate of 10 °C/min under 5 ml/min dynamic air atmosphere.
2.6.6. Heavy metal removal assay
To study the efficiency of heavy metals removal property, 100 mg of bacterial alginate beads were added to 50 ml distilled water containing 100 mg of lead nitrate, cobalt chloride and potassium dichromate. The reaction was preceded for 24 h at ambient temperature (27 ± 2 °C) under shaking at 200 rpm. The residual metal ions were dissolved in 10 ml of 2 % nitric acid, filtered with Whatmann filter paper and heated at 80 °C until the solution become transparent. The resulting solution was filtered again and diluted to 50 ml with double distilled water. Final metal concentrations were analyzed by Atomic absorption spectroscopy (AAS).
2.6.7. Gel formation assay
To test the gel formation property, harvested bacterial alginate was mixed with ion bond forming chemicals such as ZnSO4, K2Cr2O7, KMnO4 and CuSo4 [21]. For this assay, 250ul of NaOH (30 %) was mixed with 5 ml of above ionic solutions (0.16 %) followed by addition of 500ul of bacterial alginate (0.6 %) and the mixture were assessed for gel formation ability.
2.6.8. Biocompatibility evaluation
For cytotoxicity studies, Mouse fibroblast L929 (NCTC clone 929) cell line established from mouse connective tissue cells were used. In brief, the cells were cultured in Eagle’s MEM supplemented with 10 % fetal calf serum. The bacterial alginate was dissolved in α-MEM (20 mg/ml) and filter sterilized. Two fold serial dilutions were made using 2x complete medium (α-MEM, Fetal bovine serum and penicillin/streptomycin). Then, the culture was replaced with medium containing bacterial alginate (5 mg/ml and 10 mg/ml) and incubated for 24 h. Medium without bacterial alginate and Phenol (1.3 mg/ml) were used as negative and positive control respectively. After 24 h, phase contrast images were taken and the cytotoxicity was assessed as per ISO 109935.
3. Results and discussion
3.1. Characterization of isolate
3.1.1. Gram-staining and biochemical characterization
From ten isolates screened, we found one isolate significantly synthesized exopolysaccharide, which was used for further characterization. The preliminary screening of alginate producing bacteria showed Gram negative rod shape (Fig. 1) in Gram staining. The biochemical analysis of the alginate producing isolate exhibited positive for catalase, oxidase, Voges Proskauer, mannitol fermentation, nitrate reduction, starch hydrolysis, caesin hydrolysis. Further this isolate showed negative for methyl red, indole, hydrogen sulphide, urease, citrate utilization, and glucose fermentation (Table 1). These physiological and biochemical characteristics results suggested that the isolate belongs to the genus Pseudomonas.
Fig. 1.
Light microscopic image of Gram negative rod bacteria (Pseudomonas stutzeri).
Table 1.
Shows the biochemical test results of G–ve rod bacterial isolate from soil sample.
| Name of the biochemical test | Gram–Ve isolate |
|---|---|
| Catalase test | + |
| Oxidase test | + |
| Methyl red test | _ |
| Voges-proskauer test | + |
| Indole test | _ |
| Mannitol fermentation test | + |
| H2s production test | _ |
| Citrate utilization test | _ |
| Starch hydrolysis | + |
| Casein hydrolysis | + |
| Glucose fermentation | |
| Gas production | _ |
| Acid production | _ |
3.1.2. Confirmation of the spp identity of P. stutzeri by 16S rRNA sequence analysis
The DNA sequence encoding 16S rRNA is used as a molecular marker for the identification of bacterial population [22]. In the present study, 890 bp DNA sequence containing 690 bp of 16S rRNA sequence was amplified by PCR from the genomic DNA of the isolate. Sequence analysis revealed that this isolate was closely related to the genus Pseudomonas spp. BLAST analysis of the 16S rRNA sequence showed 99 % similarity to 16S ribosomal RNA gene (GenBank accession # KY941131.1) of P. stutzeri strain T2, and this strain has proven to produce biosurfactant [23,24]. To analyze the phylogenetic relationship of the isolated P. stutzeri, the programs PhyML 3.0 aLRT and Tree Dyn 198.3 were used for phylogeny analysis and tree rendering process respectively (Fig. 2) [25]. The sequence has been submitted to NCBI (GenBank accession number: MH316120.1). Based on the 16S rRNA sequence analysis and its similarity with the previously known P. stutzeri sequence, we name this strain as P. stutzeri TN_AlgSyn. The molecular identification of the isolate P. stutzeri TN_AlgSyn suggests that this strain belongs to the phylum Proteobacteria under the family of Pseudomonadaceae and P. stutzeri subgroup.
Fig. 2.
Phylogenetic view of the isolate Pseudomonas stutzeri according to partial sequence of 16S rRNA.
P. stutzeri is a rod shape, gram –ve, nonfluorescent bacterium belonging to the family of Pseudomonadaceae, and widely distributed in the environmental samples [[26], [27], [28]]. Recently, this species is receiving much attention because of its unique metabolic properties such as denitrification, degradation of aromatic compounds, and nitrogen fixation. It serves a model organism for denitrification studies; many strains have natural transformation property and others can able to degrade pollutants or interact with toxic metals.
3.1.3. Synthesis of EPS by Pseudomonas stutzeri
As described in the methods section, P. stutzeri were grown in growth medium buffered with and without MOPS and compared the exopolysaccharide production. The EPS concentration was about 7.1 g/250 ml and 3.4 g/250 ml, when the bacteria was cultured in with and without MOPS, respectively. Also, we observed that iron concentration in the growth medium significantly altered the physical properties of the EPS (Fig. 3). As shown in Fig. 3A,B, the exopolysaccharide production in medium I with MOPS and FeSO4 is clear and discrete, whereas in medium II, use of FeCl3 gives colored polymer that are found to be clumped Fig. 3C. FeSO4 has high redox potential in maintaining the pH and the microbe might not have preferred FeCl3 for its alginate production [29,30]. It could be due to alteration in pH, which lowers iron solubility and limiting the iron concentration of the growth medium or lack of siderophores (unable to bind and transport irons) in P. stutzeri. Collectively, optimum EPS production was observed at 22 days, where MOPS keep the pH constant and variation in the pH and iron could alter the physical property of bacterial EPS [29,31,32]. The synthesized alginate from medium I was used for further characterization.
Fig. 3.
EPS synthesized by P. stutzeri TN_Alg Syn using MOPS and FeSO4 (3a and 3b) EPS synthesized by P. stutzeri TN_Alg Syn without MOPS and with FeCl3 (3c).
3.1.4. Batch culture
Batch fermentation study was carried out to understand the kinetics of alginate production under shake flask cultivation conditions (Fig. 4). After 25 days, estimated biomass and alginate production were 1.7745 and 5.01 g/l, respectively. Total sugar concentration decreased to 2.0 g/l at the end of production phase in comparison to initial concentration of 20.0 g/l. Alginate yield (YP/X) was found to be 2.15 (g alginate/g biomass) with respect to substrate consumed and maximum productivity was found to be 0.2 g/l/h at 25 days. The specific growth rate (μ) was found to be 0.11 in the present study which was in correlation with previous findings of Kommedal et al. [33]. The alginate yield and productivity obtained in the current study are higher in comparison to previous findings reported in A. vinelandii where 0.928 ± 0.048 (g alginate/g biomass) and 0.281 ± 0.043 (g alginate/g biomass) was obtained using sucrose as substrate Mauricio et al. [34]. In order to deduce the production kinetics the ludking piret equation was used as follows:
Where, p represents, product concentration, x represents cell mass and α and β represented the constant terms. In the present study, the product yield followed the above equation which depicts that the rate of alginate production by isolated strain was mixed growth associated (Fig. 4).
Fig. 4.
Variation in cell mass and alginate synthesis with and different substrate (sucrose) concentration and incubation period under batch fermentation.
3.2. Characterization of bacterial alginate
3.2.1. Determination of carbohydrates
We characterized the EPS purified from P. stutzeri by various biochemical assays and we found that Molisch’s test and Iodine test showed the presence of carbohydrate from EPS synthesized by the bacteria. Seliwanoff test showed the absence of ketoses and aldoses [35]. The lack of glucose fructose, maltose and lactose from the EPS revealed the presence of other polysaccharides (Table 2). Further analysis was carried out to check the presence of alginate in EPS.
Table 2.
Shows the determination of carbohydrate obtained from P. stutzeri TN_Alg Syn harvested exopolysaccharide.
| Test | Determination of the presence of | Observation | Results (Bacterial EPS) |
|---|---|---|---|
| Molisch’s test | Carbohydrates | A deep violet coloration at the junction of two layers. | + |
| Iodine test | polysaccharide | Iodine forms coloured adsorption complexes with polysaccharides. | + |
| Seliwanoff test | Ketoses, Aldoses | A cherry red colored precipitate | – |
| Osazone Test | Glucose, Fructose, maltose and lactose | Formation of yellow crystals or Needle shaped crystalsor Hedgehog crystals or Sunflower shaped crystals | – |
3.2.2. Determination of uronic acid
Alginates are polysaccharides composed of mannuronic and guluronic acids and it becomes necessary to confirm the presence of mannuronic and guluronic acid in the EPS purified from P. stutzeri. [36] Uronic acid assay is largely used to evaluate the purity of alginate during each batch cultivation. The amount of uronic acid present in the EPS was quantitatively determined using standard alginic acid. We found that 83 ± 4 % of P. stutzeri EPS contains uronic acid. The result shows that the P. stutzeri EPS has uronic acid as its major component and remaining fractions may be presence of proteins or amino sugars and lipids [37].
3.2.3. FT-IR spectroscopic analysis
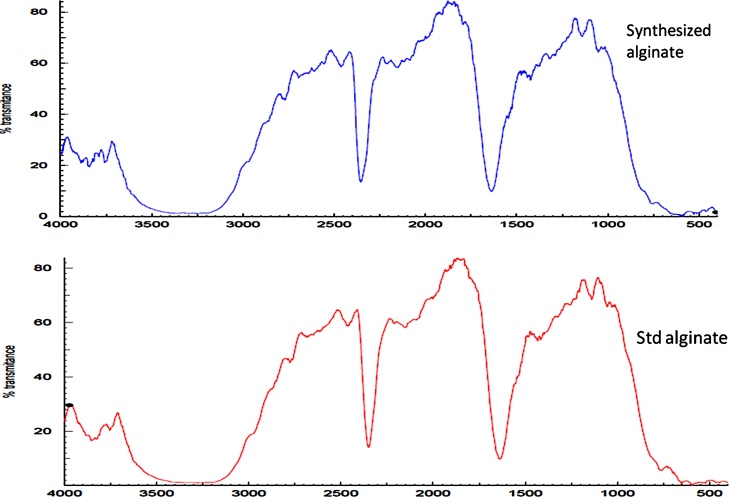
FT-IR is used to determine the functional groups present in the bacterial alginate by using typical vibration frequencies [38]. The following vibrational peaks were observed in the synthesized alginic acid sample using FTIR spectra (Fig. 5). The broad band between 3700−3000 cm−1 was assigned to OH stretching vibration and between 3000−2300 cm−1 was to C–H stretch. Alginic acid is building blocks of mannuronic and guluronic acid residues containing one −COO group [39,40]. The bands around 1650 cm−1 and 1400 cm−1 in the spectrum showed the asymmetric and symmetric stretching of carboxylate O—C—O vibration, respectively. The deformation of O—H and C—O stretching vibration were observed at 1395 cm−1 and 1333 cm−1, respectively. The bands around 1100 cm−1-1027 cm−1 was assigned to antisymmetric stretch of C—O—C. The band at 1150 cm−1 showed to the presence of o-acetyl ester in the bacterial alginates. The results of FTIR are remarkably in agreement with previous works [[41], [42], [43]]. The special characteristic feature of having dissimilar units of uronic acids made the polymer as one of the functional gel-forming exopolysaccharides [44]. Current frequency shift in the carbohydrate region strongly indicating the presence of mannuronic and guluronic acid residues.
Fig. 5.
FTIR Spectra of standard and synthesized alginate from P. stutzeri TN_Alg Syn EPS.
3.2.4. 1H NMR of bacterial alginate
NMR spectroscopy revealed the composition and block structures of bacterial alginate molecules. Based on the 1H NMR spectrum resonances, the region between 3.2–4.4 ppm up taking polysaccharides are sugar ring protons and the region from 4.4 to 5.6 up taking polysaccharides are anomeric proton [45]. The 1H spectra of isolated bacterial alginate is shown in (Fig. 6); it showed a large doublet centered peak at 4.85 ppm, which was assigned to the anomeric proton resonances of guluronic (G) and manuronic (M) acid residue, which are the two building blocks of alginate and these monomers arrangement can be in GG, MM or GM blocks distributed in a non-regular order along the alginate chain [46]. These results were similar to those published for fraction of sodium alginate from an algae Macrocystis pyrifera and bacterial alginate from P. aeruginosa respectively [47,48]. The intent signal observed between 4.2 and 5.1 ppm is assigned to alginate G5-H2 moieties of l-glucuronic acid in homopolymeric blocks [49]. The spectrum fraction of bacterial alginate also showed resonances below 3.2 ppm which are characteristics of proteins and lipids, and acetyl and succinyl groups [50,51]. In our study, the assignments of chemical shifts in the 1H NMR spectra fractions are presented in Table 3; the values were in good agreement with those published in the literature and confirmed that, these broad bands at 4.85 ppm, 3.4–3.9 ppm and 5.1 ppm are strong indicative of the presence G and M blocks of alginate.
Fig. 6.
1H NMR spectra of synthesized P. stutzeri TN_Alg alginate.
Table 3.
1H NMRspectrum fraction of bacterial alginate.
3.2.5. Differential scanning calorimetry (DSC)
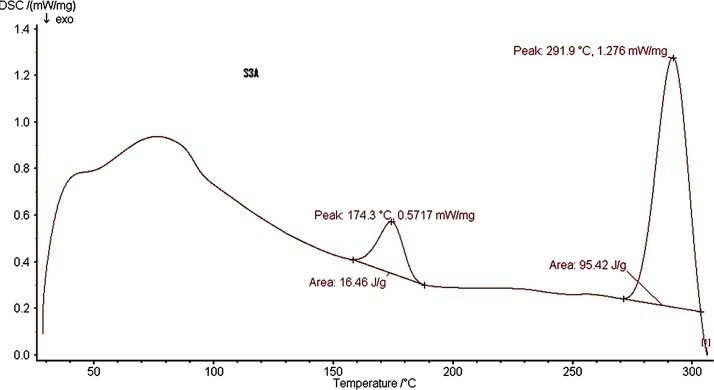
In industrial processes, the thermal (heat) analysis is an important fact regarding the information about the stability, decomposition and melting behavior of polymer [52,53]. Thermogravimetry (TG) is a simple and fast way for getting information about humidity and ash contents but the resolution of TG curve mainly depends on heat rate [54]. Soares et al. [52] compared the TG and DSC curves of sodium alginate and they concluded that the small endothermic process at 190 °C followed by a strong exothermic peak at 300 °C, corresponds to the decomposition of the biopolymer [52]. Tripathi and Mishra [55] showed that the peak shifting from 136.9 °C to 185 °C, which might be due to the addition of bulk groups (carboxylic) as a side chain to alginate and the presence of amide bonds considered for its thermal stability [55]. In our study, the DSC results showed similar pattern as previous findings [52,53,56]. Fig. 7 shows the small endothermic process at 174.3 °C followed by a strong exothermic peak at 291 °C, which could be due to pyrolysis reaction of the sample. Whereas, the biofield treatment has altered the enthalpy significantly from 16.46 J/g to 95.42 J/g. These results indicate that our isolated bacterial alginate could provide considerable thermal stability and sufficient mechanical strength during the industrial processes.
Fig. 7.
DSC curve shows thermal behavior of synthesized P. stutzeri TN_Alg alginate.
3.2.6. Heavy metal removal assay
Environmental pollutants consist of heavy metals that are toxic even at lower concentration and cause health disorders [57]. Adsorption is an efficient and cost-effective method for the removal of these heavy metals and several organic compounds including bacterial alginate have been shown to be useful in this process. In this study, we have analyzed the efficacy of synthesized bacterial alginate for the absorption of Cobalt, Chromium, and Lead. The AAS analysis showed that the alginate from P. stutzeri adsorbed the heavy metals (Fig. 8). The data shows that, the highest adsorption of lead ions (99.79 %± 0.278) compared to chromium (95.94 %± 0.264) and cobalt (96.81 %± 0.317). This finding suggests that alginate from P. stutzeri TN_AlgSyn could be a potential source for bioremediation of heavy metals.
Fig. 8.
Graph representing percentage adsorption of heavy metals by alginate from P. stutzeri TN_Alg.
3.2.7. Gel formation assay
The viscosities, gel-forming capacities, homogeneity in pore size are the most exciting properties of alginates in the field of biomedical applications. The viscosity and gel-forming capacities of alginate can be determined by the length, molar ratio and sequential distribution of M and G monomeric residues along the polymer chain [58,59]. During gel formation, divalent cations such as Na + interact with two neighbor G-blocks with ion crosslinking and form an egg-box like structure. MM or MG blocks are generally incapable of binding divalent cations. Absence of G-blocks, the length of blocks (M & G) between the links, less ability of making cross-link with ions and viscosity may contribute for gel formation. But properties of bacterial alginate can be manipulated by changing the bacterial genes and experimental conditions. Genetically modified bacteria may enable production of alginate with tailor-made features and provide wide applications in industrial and biomedical fields [4,13]. Previous finding showed that several Pseudomonas spp are unable to produce alginate with more G-blocks and researchers have shown little interest in alginate productions from them [12,58,60]. In our study, we have used four divalent cation solutions (CuSO4, ZnSO4, K2Cr2O7 and KMnO4) to test the gel-forming property of isolated P. stutzeri TN_AlgSyn alginate. P. stutzeri TN_AlgSyn alginate showed gel formation in the presence of CuSO4 and ZnSO4, whereas precipitation occurred with K2Cr2O7 and KMnO4. (Fig. 9). These results indicate that the presence of α-l-gluconate residue (G-Block) in the chain may remarkably increase the binding affinity of alginate produced by P stutzeri TN_AlgSyn with divalent cations.
Fig. 9.
Tubes show the gel formation ability of alginate from P.stutzeri TN_AlgSyn with different divalent cations.
3.2.8. Biocompatibility evaluation
The evaluation of the toxicity of bacterial alginate in L929 cells has been shown in Fig. 10. The result showed that L929 cells treated with 5 mg/ml of P. stutzeri TN_AlgSyn alginate exhibit discrete intracytoplasmatic granules without lysis. A lower number of cells with discrete morphology (not more than 10 % of the cells are rounded and loosely attached) was observed when treated with 10 mg/ml bacterial alginate. Currently alginates are being used in various biomedical applications as hydrogel and scaffold [13,61]. Here we showed that bacterial alginate synthesized from P. stutzeri TN_AlgSyn is non-cytotoxic and large scale synthesis of alginate could be cost effective source that facilitates their use in biomedical application.
Fig. 10.
Cytotoxocity evaluation of synthesized alginate in L929 cells. A. Negative control, B. Bacterial alginate 5 mg/ml, C. Bacterial alginate 10 mg/ml, D. Positive control (Phenol 1.3 mg/ml).
4. Conclusion
In the present study, we have isolated and characterized P. stutzeri TN_Alg Syn strain from natural ecological niche, which has the ability to produce alginate. The lack of uniform quality and less abundance of brown algae are limitations for large scale alginate production, and use of P. stutzeri TN_Alg Syn could overcome these limitations. Also, our results confirm that P. stutzeri TN_Alg Syn alginate is secreted as an exopolyssacharide, which has consecutive stretches of G and M blocks that can build cross link with divalent cations under desirable culture conditions. Further evaluation of pilot scale followed by large scale production L of P. stutzeri TN_Alg Syn alginate could facilitate its commercial use in bioremediation and biomedical applications. The alginate has other industrial applications such as textile, paint industries, which will be added benefits. Future studies on finding molecular mechanism of P. stutzeri TN_Alg Syn alginate synthesis could be useful for the improved alginate production.
Author statement
Alginate found to be a desirable polymer which has enormous applications. In spite of produced from marine algae, bacterial resources are promising a homogenous production. Our result justifies the integral structure of alginate through various analyses. The application of the biopolymer was justified through its biocompatibility and bio remediation. Its production details were statistically proven through batch kinetics. We here report a novel species producing alginate under laboratory conditions.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Sengha S.S., Anderson A.J., Hacking A.J., Dawes E.A. The production of alginate by Pseudomonas mendocina in batch and continuous culture. Microbiology. 1989;135(4):795–804. [Google Scholar]
- 2.Ma J.F., Phibbs P.V., Hassett D.J. Glucose stimulates alginate production and algD transcription in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 1997;148(2):217–221. doi: 10.1111/j.1574-6968.1997.tb10291.x. [DOI] [PubMed] [Google Scholar]
- 3.Bixler H.J., Porse H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011;23(3):321–335. [Google Scholar]
- 4.Hay I.D., Rehman Z.U., Moradali M.F., Wang Y., Rehm B.H. Microbial alginate production, modification and its applications. Microb. Biotechnol. 2013;6(6):637–650. doi: 10.1111/1751-7915.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid J., Sieber V., Rehm B. Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front. Microbiol. 2015;6:496–519. doi: 10.3389/fmicb.2015.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szekalska M., Puciłowska A., Szymańska E., Ciosek P., Winnicka K. Alginate: current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polymer Sci. 2016;2016:1–17. [Google Scholar]
- 7.Fabich H.T., Vogt S.J., Sherick M.L., Seymour J.D., Brown J.R., Franklin M.J., Codd S.L. Microbial and algal alginate gelation characterized by magnetic resonance. J. Biotechnol. 2012;161(3):320–327. doi: 10.1016/j.jbiotec.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalil H.P.S., Lai T.K., Tye Y.Y., Rizal S., Chong E.W.N., Yap S.W., Hamzah A.A., Fazita M.R., Paridah M.T. A review of extractions of seaweed hydrocolloids: properties and applications. Express Polymer Lett. 2018;12(4):296–371. [Google Scholar]
- 9.Zia K.M., Zia F., Zuber M., Rehman S., Ahmad M.N. Alginate based polyurethanes: a review of recent advances and perspective. Int. J. Biol. Macromol. 2015;79:377–387. doi: 10.1016/j.ijbiomac.2015.04.076. [DOI] [PubMed] [Google Scholar]
- 10.Andersen T., Strand B.L., Formo K., Alsberg E., Christensen B.E. Vol. 37. 2011. Alginates as biomaterials in tissue engineering; pp. 227–258. (Carbohydrate Chemistry. Chemical and Biological Approaches). [Google Scholar]
- 11.Peteiro C. Alginates and Their Biomedical Applications. 2018. Alginate production from marine macroalgae, with emphasis on kelp farming. ISBN: 978-981-10-6909-3. 11: 27-66. [Google Scholar]
- 12.Conti E., Flaibani A., O’Regan M., Sutherland I.W. Alginate from Pseudomonas fluorescens and Pseudomonas putida: production and properties. Microbiology. 1994;140(5):1125–1132. [Google Scholar]
- 13.Lee K.Y., Mooney D.J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cappuccino J.G., Sherman N. 9th ed. Pearson Benjamin Cummings; San Francisco: 2011. Microbiology: A Laboratory Manual. [Google Scholar]
- 15.Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talavera G., Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007;56(4):564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 17.Peña C., Hernández L., Galindo E. Manipulation of the acetylation degree of Azotobacter vinelandii alginate by supplementing the culture medium with 3‐(N‐morpholino)‐propane‐sulfonic acid. Lett. Appl. Microbiol. 2006;43(2):200–204. doi: 10.1111/j.1472-765X.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 18.Priego R., Peña C., Ramírez O.T., Galindo E. Specific growth rate determines the molecular weight of the alginate produced by Azotobacter vinelandii. Biochem. Eng. J. 2005;25:187–193. [Google Scholar]
- 19.Harley J.P., Prescot L.M. 5th ed. The Mac GrawHill Companies; 2002. Laboratory Exercises in Microbiology. ISBN-13: 978-0072320411. [Google Scholar]
- 20.Bitter T. A modified uronic acid carbazole reaction. Anal. Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 21.Auhim H.S., Hassan S.S. Production and characterization of alginate from Azotobacter vinellandii A3. Int. J. Adv. Pharm. Biol. Chem. 2013;2:507–512. [Google Scholar]
- 22.Chèneby D., Philippot L., Hartmann A., Hénault C., Germon J.C. 16S rRNA analysis for characterization of denitrifying bacteria isolated from three agricultural soils. FEMS Microbiol. Ecol. 2000;34(2):121–128. doi: 10.1111/j.1574-6941.2000.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 23.Suthar M.P., Hajoori M.A., Chaudhari R.R., Desai S.A. Isolation, screening and characterization of potent biosurfactant producing bacteria from oil contaminated site. Biosci. Discovery. 2017;8(3):301–632. [Google Scholar]
- 24.Kaya T., Aslim B., Kariptaş E. Production of biosurfactant by Pseudomonas spp. isolated from industrial waste in Turkey. Turk. J. Biol. 2014;38(3):307–317. [Google Scholar]
- 25.Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J.F., Guindon S., Lefort V., Lescot M., Claverie J.M. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36(2):465–469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palleroni N.J., Doudoroff M., Stanier R.Y., Solanes R.E., Mandel M. Taxonomy of the aerobic pseudomonads: the properties of the Pseudomonas stutzeri group. Microbiology. 1970;60(2):215–231. doi: 10.1099/00221287-60-2-215. [DOI] [PubMed] [Google Scholar]
- 27.Stewart G.J., Sinigalliano C.D., Garko K.A. Binding of exogenous DNA to marine sediments and the effect of DNA sediment binding on natural transformation of Pseudomonas stutzeri strain ZoBell in sediment columns. FEMS Microbiol. Lett. 1991;85(1):1–8. [Google Scholar]
- 28.Lalucat J., Bennasar A., Bosch R., García-Valdés E., Palleroni N.J. Biology of Pseudomonas stutzeri. Microbiol. Mol. Biol. Rev. 2006;70(2):510–547. doi: 10.1128/MMBR.00047-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horan N.J., Jarman T.R., Dawes E.A. Effects of carbon source and inorganic phosphate concentration on the production of alginic acid by a mutant of Azotobacter vinelandii and on the enzymes involved in its biosynthesis. Microbiology. 1981;127(1):185–191. [Google Scholar]
- 30.Clementi F., Fantozzi P., Mancini F., Moresi M. Optimal conditions for alginate production by Azotobacter vinelandii. Enzyme Microb. Technol. 1995;17(11):983–988. [Google Scholar]
- 31.Peña C., Campos N., Galindo E. Changes in alginate molecular mass distributions, broth viscosity and morphology of Azotobacter vinelandii cultured in shake flasks. Appl. Microbiol. Biotechnol. 1997;48(4):510–515. [Google Scholar]
- 32.Skjåk-Bræk G., Zanetti F., Paoletti S. Effect of acetylation on some solution and gelling properties of alginates. Carbohydr. Res. 1989;185(1):131–138. [Google Scholar]
- 33.Kommedal R., Bakke R., Dockery J., Stoodley P. Modelling production of extracellular polymeric substances in a Pseudomonas aeruginosa chemostat culture. Water Sci. Technol. 2001;43(6):129–134. [PubMed] [Google Scholar]
- 34.Trujillo-Roldán M., Peña C., Ramírez O.T., Galindo E. The effect of oscillating dissolved tension upon the kinetics of growth, alginate production and molecular weight in cultures of Azotobacter vinelandii. Biotechnol. Progress. 2001;17:1042–1048. doi: 10.1021/bp010106d. [DOI] [PubMed] [Google Scholar]
- 35.Brown J.H. 1939. Bergey’s Manual of Determinative Bacteriology. [Google Scholar]
- 36.Cesaretti M., Luppi E., Maccari F., Volpi N. A 96-well assay for uronic acid carbazole reaction. Carbohydr. Polym. 2003;54(1):59–61. [Google Scholar]
- 37.Anastassiou E.D., Mintzas A.C., Kounavis C., Dimitracopoulos G. Alginate production by clinical nonmucoid pseudomonas aeruginosa strains. J. Clin. Microbiol. 1987;25(4):656–659. doi: 10.1128/jcm.25.4.656-659.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chandıa N.P., Matsuhiro B., Vásquez A.E. Alginic acids in lessonia trabeculata: characterization by formic acid hydrolysis and FT-IR spectroscopy. Carbohydr. Polym. 2001;46(1):81–87. [Google Scholar]
- 39.Chandía N.P., Matsuhiro B., Mejías E., Moenne A. Alginic acids in Lessonia vadosa: partial hydrolysis and elicitor properties of the polymannuronic acid fraction. J. Appl. Phycol. 2004;16(2):127–133. [Google Scholar]
- 40.Zailanie K. Study of alginate sargassum filipendula with FTIR confirmation. J. Life Sci. Biomed. 2015;5(6):167–170. [Google Scholar]
- 41.Mackie W. Semi-quantitative estimation of the composition of alginates by infra-red spectroscopy. Carbohydr. Res. 1971;20(2):413–415. doi: 10.1016/s0008-6215(00)81397-x. [DOI] [PubMed] [Google Scholar]
- 42.Dekamin M.G., Ilkhanizadeh S., Latifidoost Z., Daemi H., Karimi Z., Barikani M. Alginic acid: a highly efficient renewable and heterogeneous biopolymeric catalyst for one-pot synthesis of the Hantzsch 1,4-dihydropyridines. RSC Adv. 2014;100(4):56658–56664. [Google Scholar]
- 43.Guo W., Wang S., Cao M., Geng W., Song C. Synthesis and characterization of alginate oligosaccharides produced by Pseudomonas mendocina NK-01. Sheng Wu Gong Cheng Xue Bao. 2009;25(9):1366–1370. [PubMed] [Google Scholar]
- 44.Van H., Busscher C.G., de Vos P. Fourier transform infrared spectroscopy studies of alginate–PLL capsules with varying compositions. J. Biomed. Mater. Res. Part A. 2003;67(1):172–178. doi: 10.1002/jbm.a.10086. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Gil G., Thomas L., Emwas A.H., Lens P.N., Saikaly P.E. NMR and MALDI-TOF MS based characterization of exopolysaccharides in anaerobic microbial aggregates from full-scale reactors. Sci. Rep. 2015;5(14316):1–12. doi: 10.1038/srep14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis T.A., Llanes F., Volesky B., Diaz-Pulido G., McCook L., Mucci A. 1 H-NMR study of Na alginates extracted from Sargassum spp. in relation to metal biosorption. Appl. Biochem. Biotechnol. 2003;110(2):75–90. doi: 10.1385/abab:110:2:75. [DOI] [PubMed] [Google Scholar]
- 47.Martínez‐Gómez F., Encinas M.V., Matsuhiro B., Pavez J. Preparation and swelling properties of homopolymeric alginic acid fractions/poly (N‐isopropyl acrylamide) graft copolymers. J. Appl. Polym. Sci. 2015;132(32):81–91. [Google Scholar]
- 48.Vogt M., Flemming H.C., Veeman W.S. Diffusion in Pseudomonas aeruginosa biofilms: a pulsed field gradient NMR study. J. Biotechnol. 2000;77(1):137–146. doi: 10.1016/s0168-1656(99)00213-8. [DOI] [PubMed] [Google Scholar]
- 49.Jančiauskaitė U., Višnevskij Č., Radzevičius K., Makuška R. Polyampholytes from natural building blocks: synthesis and properties of chitosan-O-alginate copolymers. Chemija. 2009;20(2):128–135. [Google Scholar]
- 50.Mancuso Nichols C.A., Garon S., Bowman J.P., Raguenes G., Guezennec J. Production of exopolysaccharides by Antarctic marine bacterial isolates. J. Appl. Microbiol. 2004;96(5):1057–1066. doi: 10.1111/j.1365-2672.2004.02216.x. [DOI] [PubMed] [Google Scholar]
- 51.Seviour T., Lambert L.K., Pijuan M., Yuan Z. Structural determination of a key exopolysaccharide in mixed culture aerobic sludge granules using NMR spectroscopy. Environ. Sci. Technol. 2010;44(23):8964–8970. doi: 10.1021/es102658s. [DOI] [PubMed] [Google Scholar]
- 52.Soares J.D.P., Santos J.E., Chierice G.O., Cavalheiro E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclética Química J. 2004;29(2):57–64. [Google Scholar]
- 53.Trivedi M., Patil S., Shettigar H., Bairwa K., Jana S. Spectroscopic characterization of chloramphenicol and tetracycline: an impact of biofield treatment. Pharm. Anal. Acta. 2015;6(7):395–401. [Google Scholar]
- 54.Cavalheiro E.T.G., Ionashiro M., Breviglieri S.T., Marino G., Chierice G.O. The influence of experimental factors on the results of thermogravimetric analyzes. Química Nova. 1995;18(3):305–308. [Google Scholar]
- 55.Tripathi R., Mishra B. Development and evaluation of sodium alginate–polyacrylamide graft–co-polymer-based stomach targeted hydrogels of famotidine. AAPS PharmSciTech. 2012;13(4):1091–1102. doi: 10.1208/s12249-012-9824-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kulig D., Zimoch-Korzycka A., Jarmoluk A., Marycz K. Study on alginate–chitosan complex formed with different polymers ratio. Polymers. 2016;8(5):167–183. doi: 10.3390/polym8050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parmar M., Thakur L.S. Heavy metal Cu, Ni and Zn: toxicity, health hazards and their removal techniques by low cost adsorbents: a short overview. Int. J. Plant Anim. Environ. Sci. 2013;3(3):143–157. [Google Scholar]
- 58.Sabra W., Zeng A.P., Deckwer W.D. Bacterial alginate: physiology, product quality and process aspects. Appl. Microbiol. Biotechnol. 2001;56(4):315–325. doi: 10.1007/s002530100699. [DOI] [PubMed] [Google Scholar]
- 59.Matricardi P., Di Meo C., Coviello T., Hennink W.E., Alhaique F. Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Delivery Rev. 2013;65(9):1172–1187. doi: 10.1016/j.addr.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Draget K.I., Taylor C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocolloids. 2011;25(2):251–256. [Google Scholar]
- 61.Rokstad A.M., Brekke O.L., Steinkjer B., Ryan L., Kolláriková G., Strand B.L., Skjåk-Bræk G., Lacík I., Espevik T., Mollnes T.E. Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a human whole blood model. Acta Biomater. 2011;7(6):2566–2578. doi: 10.1016/j.actbio.2011.03.011. [DOI] [PubMed] [Google Scholar]