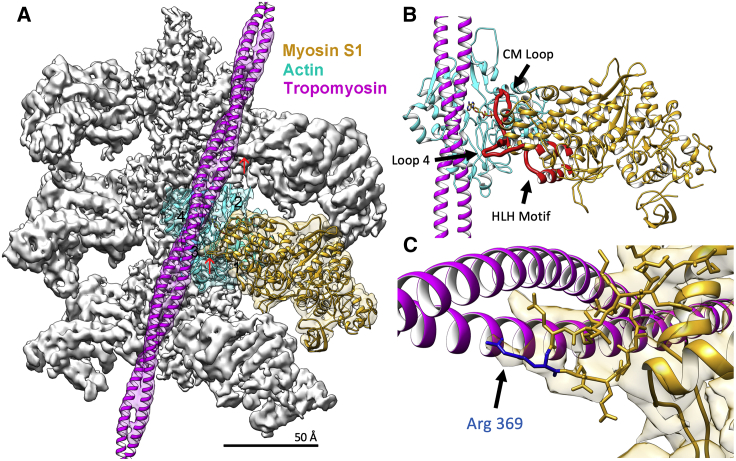
Figure 1.
Cryo-EM reconstruction of S1-decorated actin-tropomyosin filaments. (A) Isosurface rendering of the reconstruction showing atomic models of actin (cyan), myosin (gold), and tropomyosin (magenta) fitted into respective EM densities as described in the Materials and Methods. The pointed end of the filament is facing up; actin subdomains numbered on central actin subunit. Each region of the reconstruction was low-pass filtered to provide better visualization; actin to 4.0 Å, myosin to 5.5 Å, and tropomyosin to 6.5 Å according to local resolution estimates (see Fig. S2). (B) Atomic model of the actin-myosin-tropomyosin complex highlighting the actin-myosin interface. Extensive interactions are formed between actin and the myosin cardiomyopathy loop (CM Loop), loop 4, and the helix-loop-helix (HLH) motif (each highlighted in red). ADP bound to actin is shown. The residue-to-residue contacts between actin and myosin are tabulated in Table S3. (C) Myosin-tropomyosin interaction is visible at myosin loop 4. The cryo-EM density of this region is well resolved, allowing identification of interacting amino acid side chains of myosin, including that of conserved residue Arg369 (blue) making contact with the tropomyosin coil-coil (also noted by red arrows in (A)). To see this figure in color, go online.