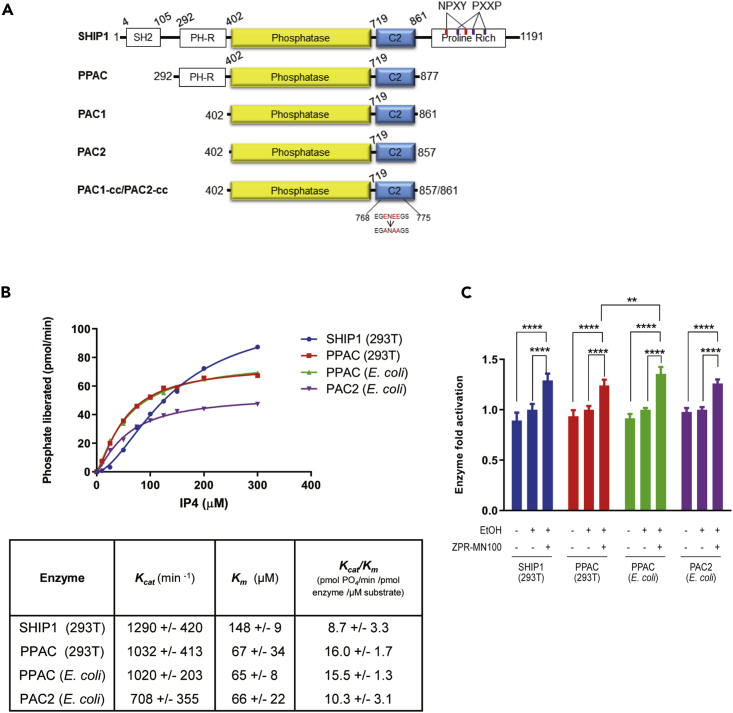
Figure 5.
PPAC, PAC1, and PAC2 Have Similar Enzymatic Activity as Full-Length SHIP1
(A) Schematic diagram of the different SHIP1 truncation constructs. PPAC consists of PH-R domain, phosphatase, and C2 domain (residues aa 293-877). PAC1 and PAC2 consists of phosphatase and C2 domain (residues aa 402-861 and aa 402-857, respectively). PAC1-cc and PAC2-cc contain surface entropy reduction mutations in C2 domain (E770A, E772A, E773A). This cluster of residues was identified using the SERp server (http://services.mbi.ucla.edu/SER/intro.php).
(B) Enzyme catalytic initial velocities were determined at the indicated concentrations of IP4. Kcat and Km values were calculated using GraphPad software.
(C) Ability of ZPR-MN100 to stimulate phosphatase activity in full-length SHIP1, PPAC, and PAC (Data represent means ± SD. Two-way ANOVA with Tukey correction for multiple comparisons, ∗∗p < 0.01, ∗∗∗∗p < 0.0001).