Abstract
The human gut microbiome and its metabolite Trimethylamine N-oxide (TMAO) are sensitive to the human diet and are involved in the complex pathomechanisms that underpin diabetes, obesity, and cardiovascular diseases. A potential involvement of increased TMAO in atrial fibrillation (AF) manifestation and progression is not clear. We measured TMAO in peripheral blood of 45 AF patients and 20 non-AF individuals (matched for age, sex, BMI, prevalence of hypertension and diabetes). TMAO levels in AF (median [IQR] 3.5 µM [2.51–4.53]) were comparable with those in non-AF individuals (3.62 µM [2.49–5.46]) (p = 0.629). There was no association between TMAO and AF progression phenotypes (p = 0.588). In 35 AF patients, TMAO was additionally measured 12–18 months after AF catheter ablation. TMAO levels at baseline and follow-up were correlated (r = 0.481, p = 0.003), and TMAO was increased independent from the success (restoration of sinus rhythm) of the ablation procedure.
The data of this pilot study indicate that TMAO is not generally higher in AF and is not associated with AF progression phenotypes. The observed TMAO increase 12–18 months after AF catheter ablation needs further investigation in a larger cohort.
Keywords: Atrial fibrillation, Trimethylamine N-oxide, Atrial fibrillation progression, Recurrences
1. Introduction
The human gut microbiome catabolizes dietary L-carnitine, choline and lecithin into trimethylamine, which is converted into trimethylamine N-oxide (TMAO) in the liver. Increased TMAO is associated with cardiovascular disease such as coronary artery disease, congestive heart failure, atherosclerosis, acute myocardial infarction, and the related cardiovascular mortality [1]. Atrial fibrillation (AF) is the most common clinical arrhythmia with the general risk factors - older age, hypertension and obesity - of other cardiovascular diseases. AF is considered as a progressive disease characterized by a switch from paroxysmal (PAF) to persistent (persAF) AF and structural left atrial remodeling that can be detected peri-interventionally as low voltage areas (LVA).[2] Advanced progression stages are associated with higher levels of stress biomarkers like NT-proANP [2].
TMAO injection into atrial ganglionated plexi in healthy dogs undergoing rapid atrial pacing resulted in increased neural activity, electrical remodeling and activation of the proinflammatory NF-κB pathway leading to aggravated autonomic remodeling and AF progression.[3] We hypothesized that TMAO level increase may underpin AF progression, which has not been analyzed yet, and addressed this hypothesis in current study.
2. Methods
2.1. AF patients and non-AF controls
Consecutively selected patients with symptomatic non-valvular AF undergoing first AF radiofrequency catheter ablation were recruited at the Heart Center Leipzig in 2015–2017. Exclusion criteria were pregnancy, age < 18 or > 75 years, cancer, acute or systemic inflammatory diseases and renal dysfunction with estimated glomerular filtration rate (eGFR) < 15 ml/min/1.73 m2 or dialysis. Catheter ablation procedure with isolation of the pulmonary veins and additional lesions if required was performed as reported previously [2]. Briefly, the electro-anatomical mapping was performed in sinus rhythm that was achieved by electrical cardioversion if necessary. End-point of the catheter ablation was isolation of the pulmonary veins with proof of both exit and entrance block. The cut-off values for defining LVA were < 0.5 mV for low voltage areas. Paroxysmal AF was defined as self-terminating within 7 days after onset. Persistent AF lasted longer than 7 days or required drugs and/or electrical cardioversion for termination. AF progression phenotypes were defined in accordance to AF type (PAF or persAF) and presence/absence of LVA.
Patients from the cardiology outpatient clinic at the Heart Center Leipzig were recruited in 2018 as controls. If clinical, ECG and echocardiography data indicated any cardiovascular pathology requiring further diagnostics and treatment (e.g. any history of cardiac disease (coronary artery disease, valve disease ≥ 2nd degree, cardiomyopathy, acute coronary syndrome, unstable angina pectoris, heart failure, any history of myocardial infarction, thromboembolic events, and supraventricular/ventricular arrhythmia), or cancer, severe renal impairment (eGFR < 60 ml/min/1.73 m2), liver dysfunction, acute or systemic infections, and autoimmune disease), individuals were not included as controls.
The responsible institutional ethics board of the Medical Faculty of the University of Leipzig approved both studies, and all patients provided written informed consent for participation in accordance with the Declaration of Helsinki.
2.2. Follow‑up
During the follow-up (FU) period, 4-day Holter ECG recordings were performed at 3, 6, and 12 months after ablation. Additional ECGs and Holter ECG recordings were obtained if patients’ symptoms were suggestive of AF. AF recurrences were defined as any atrial arrhythmia lasting > 30 sec. If electrical or pharmacologic cardioversion and/or repeat ablation were needed after 3 months blanking period, this was considered as an AF recurrence. In 35 patients, blood was collected 12–18 months after ablation procedure during FU.
2.3. Determination of TMAO
TMAO was measured using electrospray ionization tandem mass spectrometry according to a modified protocol of Wang et al. [4]. The modifications are described by Schneider et al. [4]. In brief, plasma samples were prepared according to Wang et al. with minor modifications: 10 µL samples were pipeted into a 1.5 ml centrifuge tube. After adding 340 µL of a cold (4 °C) mixture of Methanol (MeOH) and Acetonitrile (ACN; MeOH:ACN 25:75, v/v) and 50 µL of a 2 µM D9-Trimethylamine-N-oxide (Cambridge Isotopes Laboratories, Tewksbury, MA, USA) solution in MeOH, the protein was precipitated by vortex-mixing for 30 sec followed by 10 min incubation. The mixture was centrifuged for 5 min at 18.000 × g and 150 µL of the supernatant were transferred to a 96-well microplate. Samples were quantified by an external 9-point calibration using the peak area ratio of TMAO towards D9-TMAO. For calibrators, fetal bovine serum (FBS) was used as matrix. After spiking with TMAO in the range between 0 – 200 µM, calibrators were prepared as described above. In addition, three quality controls (3 µM, 15 µM, and 75 µM in FBS) were included in each sample sequence. When all calibrators, quality controls and samples of a sequence were transferred, the microplate was sealed with a preslit adhesive foil.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses were performed using a Waters XEVO TQS system (Waters, Eschborn, Germany) equipped with an electrospray ion source. The instrument was controlled with MassLynx 4.1 (Waters Corporation, Milford, MA, USA) software. For chromatographic separation, a HILIC (Hydrophilic Interaction Chromatography) column (Waters Acquity UPLC BEH Amide 100 × 2.1 mm; 1.7 um) with a corresponding pre-column (Waters Acquity UPLC BEH Amide VanGuard, 5 × 2.1 mm; 1.7 µM) was used in isocratic mode. During a 3 min chromatographic run, eluent A (10 mM ammonium formate in ultrapure water (H2OmQ) and ACN (H2O mQ:ACN 95:5, v/v)) and eluent B (ACN) were applied with a mixing ration of 42% A and 58% B at a flow rate of 0.4 ml/min. The injection volume was 1 µL. The analytes were analyzed using a multiple reaction monitoring (MRM) experiment containing their most abundant mass transitions: (TMAO: 76.1 Da → 59.1 Da; d9-TMAO: 85.1 Da → 68.1 Da; cone voltage: 40 V; collision energy: 11 V) in positive ion mode at a flow rate of 0.4 ml/min for 3 min.
2.4. Statistics
A power calculation was done prior the study. Our setting with 20 non-AF individuals and 45 AF patients allowed for the detection of an effect size > 0.70 (α = 0.05 and β = 0.8). Continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test. The differences between continuous values were assessed using Mann-Whitney U test (two groups), Kruskal Wallis test (more than two groups) and Wilcoxon exact test was used for before-after ablation comparisons. Chi-square test was used for categorical variables. Correlations were analyzed using Pearson correlation. Linear regression analysis was used to analyze the relationship between continuous data. A p-value < 0.05 was considered as statistically significant. Statistics and visualization were performed using IBM SPSS Statistics for Windows Version 26 (IBM Corp, Armonk, NY) and GraphPad Prism 8 (GraphPad Software, San Diego, CA).
3. Results
TMAO was analyzed in peripheral blood of 45 AF patients (65 ± 10 years, 60% women, 89% hypertension, 22% diabetes, body mass index (BMI) 30 ± 5 kg/m2, 47% LVA) undergoing first radiofrequency catheter ablation and 20 non-AF individuals (65 ± 9 years, 45% women, 85% hypertension, 25% diabetes, BMI 30 ± 6 kg/m2).
There were 12 patients with PAF without LVA, 12 had PAF with LVA, 12 had persAF without LVA, and 9 had persAF with LVA (Table 1).
Table 1.
Clinical characteristics of study cohort and concentration of TMAO (µM).
| AF | Non-AF controls | p-value | PAF w/o LVA | persAF w/o LVA | PAF with LVA | persAF with LVA | p-value | |
|---|---|---|---|---|---|---|---|---|
| n | 45 | 20 | 12 | 12 | 12 | 9 | ||
| Age (years) | 65 ± 10 | 65 ± 9 | 0.695 | 63 ± 9 | 61 ± 13 | 69 ± 7 | 70 ± 6 | 0.142 |
| Women, n (%) | 27 (60) | 9 (45) | 0.291 | 9 (75) | 6 (50) | 7 (58) | 5 (56) | 0.634 |
| BMI (kg/m2) | 30 ± 4.5 | 30 ± 5.9 | 0.936 | 31 ± 5.2 | 29 ± 3.6 | 30 ± 3.8 | 32 ± 5.3 | 0.538 |
| eGFR (ml/min/1.73) m2) | 76 ± 16 | na | na | 80 ± 17 | 76 ± 16 | 69 ± 26 | 72 ± 8 | 0.439 |
| Hypertension, n (%) | 40 (89) | 17 (85) | 0.693 | 10 (83) | 10 (83) | 12 (1 0 0) | 8 (89) | 0.522 |
| ACE inhibitors / ARB, n (%) | 33 (73) | 13 (68) | 0.690 | 9 (75) | 6 (50) | 10 (83) | 8 (89) | 0.445 |
| Statins, n (%) | 8 (18) | 5 (26) | 0.438 | 0 | 0 | 7 (58) | 1 (11) | 0.074 |
| Diabetes, n (%) | 10 (22) | 5 (25) | 1.000 | 5 (42) | 4 (33) | 0 (0) | 1 (11) | 0.056 |
| Vascular disease, n (%) | 9 (20) | nd | na | 1 (8) | 0 (0) | 6 (50) | 2 (22) | 0.013 |
| TMAO (µM) | 3.7 ± 1.6 | 4.2 ± 2.4 | 0.308 | 3.6 ± 1.4 | 4.0 ± 1.7 | 3.6 ± 2.0 | 3.3 ± 0.9 | 0.741 |
Data are provided as mean +/− standard deviation or n (%).
Abbreviations: ACE – angiotensin converting enzyme, AF – atrial fibrillation, ARB – Angiotensin II receptor blockers, PAF – paroxysmal atrial fibrillation, persAF – persistent atrial fibrillation, LVA – low voltage areas, eGFR – estimated glomerular filtration rate, BMI – body mass index, TMAO – Trimethylamine N-oxide.
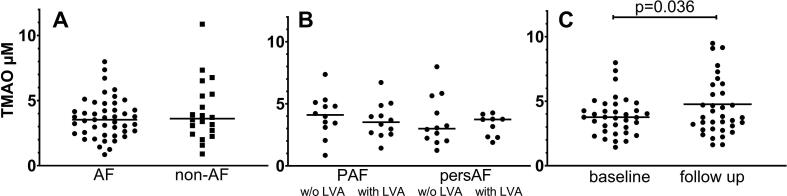
No differences were observed in age, BMI, sex, and the proportion of diabetes and hypertension, the use of anti-hypertensive medication or statins between AF and non-AF groups (Table 1). TMAO level in AF (median [IQR], 3.53 µM [2.51–4.53]) and in non-AF individuals (3.62 µM [2.49–5.46]) were similar (p = 0.629) (Fig. 1 panel A). TMAO levels were not associated with age, BMI, sex, diabetes or vascular disease (summary of coronary artery disease, peripheral vascular disease, and atherosclerotic plaques).
Fig. 1.
Plasma TMAO (µM) in A– patients with atrial fibrillation (AF) or without AF (non-AF), B – AF patients at different AF progression stages (paroxysmal AF (PAF) with/ without (w/o) low voltage areas (LVA) or persistent AF (persAF), C – AF patients before (baseline) and after AF catheter ablation (follow up).
In AF patients TMAO was significantly (p = 0.005) higher in patients with hypertension (3.75 µM [2.71–4.80] vs. 1.89 µM [1.14–2.89]). This association was not observed in the control group (hypertension 3.92 µM [IQR estimation not applicable due to low sample number, n = 3] vs. no hypertension 3.52 µM [2.57–5.42], p = 0.874).
An inverse correlation between eGFR and TMAO (r = -0.401, B = -0.038, p = 0.014) was found in AF patients at baseline and at FU (r = -0.510, B = -0.096, p = 0.009). eGFR at baseline and FU was comparable (74 [66–88] vs. 82 [66–91] ml/min/1.73 m2, respectively, p = 0.965).
Analyzing the association between TMAO plasma concentration and AF progression, no differences (p = 0.588) were found between the four phenotype groups: PAF without LVA (3.5 µM [2.58–4.66]), PAF with LVA (3.0 µM [2.07–5.30]), persAF without LVA (4.1 µM [3.19–5.04]), and persAF with LVA (3.7 µM [2.32–3.97]) (Fig. 1 panel B). In 35 AF patients with available blood samples at 12–18 months FU, TMAO was significantly increased (3.9 ± 1.5 vs. 4.8 ± 2.8 µM, respectively, p = 0.036) whereas TMAO levels at baseline and FU were moderately but significantly correlated (r = 0.481, p = 0.003) (Fig. 1 panel C). The TMAO increase was numerically independent from the success of the ablation procedure (sinus rhythm restoration: n = 21, baseline 3.6 ± 1.1 vs. FU 4.6 ± 2.2 µM (p = 0.058), AF recurrence: n = 14, baseline 4.3 ± 1.9 µM vs. FU 5.0 ± 3.6 µM (p = 0.324)). When comparing patients with recurrent AF and patients in sinus rhythm neither baseline (p = 0.377) nor FU TMAO concentrations (p = 0.881) differed.
4. Discussion
We found that TMAO levels in 45 AF patients and 20 non-AF controls were similar and comparable with published ranges determined in 349 healthy Chinese (3.45 µM [2.25–5.79])[5] and 100 healthy Europeans (women 3.6 µM [2.9–4.7] and men 3.7 µM [2.6–4.6]) [6]. These results contradict prior findings in patients from the Western Norway Coronary Angiography Cohort (WECAC), where AF incidence (10.9%) during a 7 years FU correlated with higher baseline TMAO [7]. Noteworthy, an estimate of about 50% of the WECAC patients had TMAO level above 5 µM and 25% had level around 15.8 µM, whereas in our cohort only 18% of the AF patients had TMAO level above 5 µM with 8 µM as highest measurement. In contrast to our clinical AF cohort, the WECAC observational cohort included a high percentage of patients with previous cardiovascular diseases, e.g. > 40% of patients with previous acute myocardial infarction and > 26% with prior coronary revascularization. In addition in WECAC cohort TMAO was positively associated with the extend of coronary artery disease determined at angiography [7]. Importantly, coronary heart disease is a strong and independent risk factor for AF [8]. We thus assume that increased AF incidence in the WECAC cohort may merely be a result of primary cardiac disease and cardio-vascular risk factors and that the observed high TMAO level are an independent characteristic of maybe unrecognized cardiovascular pathomechanisms that are not present in our AF cohort.
We found a positive association of TMAO with hypertension and an inverse correlation with eGFR. TMAO is cleared by the kidneys and thus impaired renal function results in higher TMAO level [9]. The link between cardiovascular diseases and hypertension is supposed to be mediated by gut metabolites other than TMAO, the short-chain fatty acids [10], which were not measured in this study. Additionally, hypertension is one of the most common causes of chronic kidney disease and may thus result in impaired elimination capacity for TMAO.
Summarizing, in our pilot study TMAO levels were comparable between AF and non-AF individuals. Also, there was no association between TMAO levels and AF progression phenotypes. Higher TMAO level may thus be rather triggered by common AF risk factors and comorbidities such as hypertension, renal dysfunction, and coronary heart disease or yet unrecognized underpinning pathomechanisms in AF.
Finally, we observed a TMAO increase 12–18 months following catheter ablation procedure irrespective of sinus rhythm restoration. This may reflect intra-individual variation [5] and differences in the fasting state at the time of FU blood withdrawal, as all patients fasted > 8 h before catheter ablation. Worsening kidney function as a reason for increasing TMAO levels in our AF cohort is rather unlikely, as eGFR was comparable at baseline and FU. Importantly, TMAO increase may also reflect unknown underpinning progressing cardiovascular pathomechanisms as discussed above [1].
5. Limitations
Our study is based on a small sample number that is the main limitation. However, to minimize possible bias, we matched AF patients and non-AF controls in accordance to the most important cardiovascular risk factors age, sex, BMI, hypertension and diabetes. Nevertheless, small effects were not detectable in this setting. Controls were included if there was no evidence for clinically relevant cardiac and renal disease. Nevertheless, it is possible that they had subclinical cardiovascular or renal disease. The eGFR was not determined in control group and is thus not available for analysis. We cannot rule out that TMAO concentrations were affected by dietary intake of carnitine, choline and betaine, as diet of AF patients and controls was not determined. Finally, asymptomatic recurrences may have been overlooked, as continuous rhythm monitoring (e.g. implanted loop recorders) were not available in our study.
Disclosures: Dr. Kornej received funding from the Marie Sklodowska-Curie Actions under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 838259).
CRediT authorship contribution statement
Petra Büttner: Conceptualization, Validation, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration. Jürgen G. Okun: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - review & editing, Supervision, Project administration. Jana Hauke: Methodology, Validation, Investigation, Writing - review & editing. Erik Holzwirth: Validation, Investigation, Data curation, Writing - original draft, Writing - review & editing. Danilo Obradovic: Validation, Investigation, Data curation, Writing - original draft, Writing - review & editing. Gerhard Hindricks: Resources, Writing - review & editing, Supervision. Holger Thiele: Resources, Writing - original draft, Writing - review & editing, Supervision. Jelena Kornej: Conceptualization, Validation, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Supervision.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100554.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Velasquez M.T., Ramezani A., Manal A., Raj D.S. Trimethylamine N-oxide: the good, the bad and the unknown. Toxins (Basel) 2016;8 doi: 10.3390/toxins8110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Büttner P., Schumacher K., Dinov B., Zeynalova S., Sommer P., Bollmann A., Husser D., Hindricks G., Kornej J. Role of NT-proANP and NT-proBNP in patients with atrial fibrillation: association with atrial fibrillation progression phenotypes. Heart Rhythm. 2018:1132–1137. doi: 10.1016/j.hrthm.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Yu L., Meng G., Huang B., Zhou X., Stavrakis S., Wang M., Li X., Zhou L., Wang Y., Wang M., Wang Z., Deng J., Po S.S., Jiang H. A potential relationship between gut microbes and atrial fibrillation: Trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int. J. Cardiol. 2018;255:92–98. doi: 10.1016/j.ijcard.2017.11.071. [DOI] [PubMed] [Google Scholar]
- 4.Schneider C., Okun J.G., Schwarz K.V., Hauke J., Zorn M., Nürnberg C., Ungerer M., Ringleb P.A., Mundiyanapurath S. Trimethylamine-N-oxide (TMAO) Is elevated in the acute phase after ischemic stroke and decreases within the first days. Eur. J. Neurol. 2020 doi: 10.1111/ene.14253. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z., Levison B.S., Hazen J.E., Donahue L., Li X.-M., Hazen S.L. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal. Biochem. 2014;455:35–40. doi: 10.1016/j.ab.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kühn T., Rohrmann S., Sookthai D., Johnson T., Katzke V., Kaaks R., von Eckardstein A., Müller D. Intra-individual variation of plasma trimethylamine-N-oxide (TMAO), betaine and choline over 1 year. Clin. Chem. Lab. Med. 2017;55:261–268. doi: 10.1515/cclm-2016-0374. [DOI] [PubMed] [Google Scholar]
- 7.Svingen G.F.T., Zuo H., Ueland P.M., Seifert R., Løland K.H., Pedersen E.R., Schuster P.M., Karlsson T., Tell G.S., Schartum-Hansen H., Olset H., Svenningsson M., Strand E., Nilsen D.W., Nordrehaug J.E., Dhar I., Nygård O. Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation. Int. J. Cardiol. 2018;267:100–106. doi: 10.1016/j.ijcard.2018.04.128. [DOI] [PubMed] [Google Scholar]
- 8.Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B., Castella M., Diener H.-C., Heidbuchel H., Hendriks J., Hindricks G., Manolis A.S., Oldgren J., Popescu B.A., Schotten U., van Putte B., Vardas P., Agewall S., Camm J., Baron Esquivias G., Budts W., Carerj S., Casselman F., Coca A., de Caterina R., Deftereos S., Dobrev D., Ferro J.M., Filippatos G., Fitzsimons D., Gorenek B., Guenoun M., Hohnloser S.H., Kolh P., Lip G.Y.H., Manolis A., McMurray J., Ponikowski P., Rosenhek R., Ruschitzka F., Savelieva I., Sharma S., Suwalski P., Tamargo J.L., Taylor C.J., van Gelder I.C., Voors A.A., Windecker S., Zamorano J.L., Zeppenfeld K. ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18(2016):1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 9.Gruppen E.G., Garcia E., Connelly M.A., Jeyarajah E.J., Otvos J.D., Bakker S.J.L., Dullaart R.P.F. TMAO is associated with mortality: impact of modestly impaired renal function. Sci. Rep. 2017;7:13781. doi: 10.1038/s41598-017-13739-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma J., Li H. The role of gut microbiota in atherosclerosis and hypertension. Front. Pharmacol. 2018;9:1082. doi: 10.3389/fphar.2018.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.