Despite continuous improvement in our knowledge about atrial fibrillation (AF) pathophysiology, pharmacological, ablative and surgical treatments are still suboptimal, so AF remains a challenge of contemporary cardiology [1], [2], [3], [4]. Although there are many well-established predisposing conditions and risk factors for AF, there is still a clear unmet need to expand knowledge of new mechanisms and treatment strategies to reduce morbidity and mortality in AF patients.
Beyond other risk factors, the Western diet and additional lifestyle-component have been linked to the occurrence and progression of AF. Growing evidence has demonstrated that gut microbiota related mechanisms play a critical role in many cardiovascular diseases including AF [5]. The most recent and under-examined agent significantly affecting the process of initiation and progression of AF is trimethylamine N-oxide (TMAO), a microbial-derived metabolite generated in a two-step process including enzymatic transformation of dietary choline or L-carnitine into TMA and its further oxidization in the liver by flavin-containing monooxygenase (FMO) [6].
Several pathological mechanisms of TMAO in AF have been reported [5], [6], [7], [8], [9], [10]. TMAO may promote AF by modulating the cardiac autonomic nervous system [5] and cellular osmolarity [7], promoting inflammation [6], causing cardiac fibrosis [8], increasing thrombosis via platelet activation [9], and promoting coronary atherosclerosis [10].
TMAO may also have some prognostic value as a biomarker. Recently, elevated plasma levels of TMAO have been proven to be a strong predictor of AF development among patients with and without suspected stable angina [7], ischemic stroke and cardiac thrombus formation among patients with AF [11] and there is a proven correlation between TMAO levels and CHA2DS2-VASc score [12].
The precise mechanisms linking AF and TMAO in humans are still poorly understood, and most studies have focused on the predictive value of TMAO for first AF incidence. In this context, Büttner et al. [13], detected the levels of TMAO in 45 AF patients and compared them to those of 20 sinus rhythm controls (nSR), both before and after catheter ablation. The authors did not observe a significant difference in the levels of TMAO between AF patients and nSR, and detected no correlation between the levels of TMAO and AF progression phenotypes. Despite the overall increase during the 12–18-month follow-up, the TMAO levels increased independently from the success of the catheter ablation procedure, defined as restoration of nSR. These findings are inconsistent with the many positive correlations between TMAO and AF in the literature, likely because the study population was rather small and underpowered to detect a positive correlation between AF and TMAO levels. In addition, subclinical cardiovascular or renal disease might be present in the nSR group, also biasing the results. Finally, lack of detailed and longitudinal information on dietary intakes, enabling to estimate daily intake of total carnitine, choline, betaine and sodium could lead to an underestimation of true TMAO-AF relationship [14].
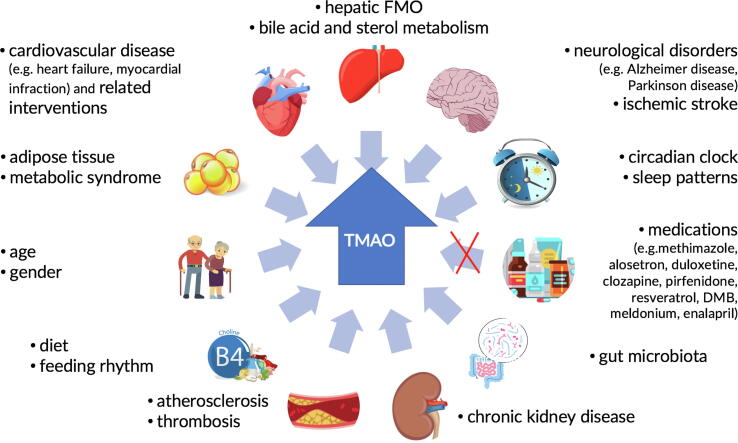
Plasma TMAO levels may show wide inter- and intra-individual variations, which could be influenced by several factors (Fig. 1). A lack of adjustment for these covariables might mask potential differences between TMAO levels, AF-type and AF-treatment. TMAO concentration increases with age, estrogens (while testosterone acts as suppressor) and levels of cholic acid that induce FMO expression [15]. Noteworthy, the gut microbiota composition and its metabolites profile are highly specific to the host and are modulated by many extrinsic (diet, lifestyle, and medication) and intrinsic (host genetics, immune, and metabolic regulations) factors [16]. More than 15% of gut microbiota community exhibits diurnal oscillations that are influenced by feeding rhythms [17] and sleep patterns [18]. Diet is thought to explain over 20% of the gut microbiota structural variations in the human, indicating the potential for therapeutic dietary strategies to manipulate microbial diversity, composition, and stability. Recently, high-fat, high-protein or rich in indigestible starch diets were shown to increase plasma TMAO levels [15]. There is also evidence that TMAO is a key regulator not only of lipid metabolism, but also of white adipose tissue formation [19]. Renal clearance plays a major and critical role in plasma TMAO levels, with a reduction in glomerular filtration rate increasing TMAO levels [20]. Further, it is important to bear in mind that medications such a methimazole, alosetron, duloxetine, clozapine, pirfenidone, which inhibit FMO, along with meldonium, resveratrol, 3,3-dimethyldimethyl-1-butanol, enalapril may all cause a reduction in the TMAO levels [15]. Several experimental studies suggest a possible involvement of TMAO in the etiology of cardiovascular disease and neurological disorders [15]. The close link between TMAO and inflammatory burden suggests that inflammation could mediate some of the deleterious effects of TMAO. However, the precise effects of cardiovascular medications on the levels of TMAO need further investigation. Finally, the assessment of TMAO plasma levels likely represent a composite consequence of diet and lifestyle components, which may result in significant temporal fluctuations. Therefore, a more longitudinal assessment of TMAO together with a diet and lifestyle diary rather than one spot-assessment may be required to better characterize the relationship to AF presentation and its progression.
Fig. 1.
Schematic representation of the impact of various intrinsic and extrinsic factors on the trimethylamine N-oxide levels.
In conclusion, TMAO appears to constitute a putative bio- and lifestyle-marker and a predictor of new-onset AF, its progression and the AF-associated ischemic events beyond traditional risk factors. Future large-scale studies comparing TMAO levels in patients with different types of AFs (paroxysmal/persistent/permanent) and TMAO fluctuations related to rhythm control strategies (cardioversion/catheter ablation) are needed to prove and validate whether TMAO and other gut microbiota-derived metabolites might represent modifiable risk factors of AF.
References
- 1.Wakili R., Riesinger L., Fender A.C., Dobrev D. Double Jeopardy: Will the new trials tell us how to manage patients with atrial fibrillation and coronary artery disease? Int. J. Cardiol. Heart Vasc. 2019;23 doi: 10.1016/j.ijcha.2019.100369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelsgaard C.S., Pedersen K.B., Riber L.P., Pallesen P.A., Brandes A. The long-term efficacy of concomitant maze IV surgery in patients with atrial fibrillation. Int. J. Cardiol. Heart Vasc. 2018;19:20–26. doi: 10.1016/j.ijcha.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang T.Y., Liao J.N., Chao T.F., Vicera J.J., Lin C.Y., Tuan T.C. Oral anticoagulant use for stroke prevention in atrial fibrillation patients with difficult scenarios. Int. J. Cardiol. Heart Vasc. 2018;20:56–62. doi: 10.1016/j.ijcha.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dan G.A., Dobrev D. Antiarrhythmic drugs for atrial fibrillation: Imminent impulses are emerging. Int. J. Cardiol. Heart Vasc. 2018;21:11–15. doi: 10.1016/j.ijcha.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishima R.S., Elliott A.D., Sanders P., Linz D. Microbiome and atrial fibrillation. Int. J. Cardiol. 2018;255:103–104. doi: 10.1016/j.ijcard.2017.12.091. [DOI] [PubMed] [Google Scholar]
- 6.Yu L., Meng G., Huang B., Zhou X., Stavrakis S., Wang M. A potential relationship between gut microbes and atrial fibrillation: Trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int. J. Cardiol. 2018;255:92–98. doi: 10.1016/j.ijcard.2017.11.071. [DOI] [PubMed] [Google Scholar]
- 7.Svingen G.F.T., Zuo H., Ueland P.M., Seifert R., Loland K.H., Pedersen E.R. Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation. Int. J. Cardiol. 2018;267:100–106. doi: 10.1016/j.ijcard.2018.04.128. [DOI] [PubMed] [Google Scholar]
- 8.Organ C.L., Otsuka H., Bhushan S., Wang Z., Bradley J., Trivedi R. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circ. Heart Fail. 2016;9(1) doi: 10.1161/CIRCHEARTFAILURE.115.002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu W., Gregory J.C., Org E., Buffa J.A., Gupta N., Wang Z. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong D., Zhang L., Zhang Y., Wang F., Zhao Z., Zhou X. Gut microbial metabolite trimethylamine N-oxide is related to thrombus formation in atrial fibrillation patients. Am. J. Med. Sci. 2019;358(6):422–428. doi: 10.1016/j.amjms.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Liang Z., Dong Z., Guo M., Shen Z., Yin D., Hu S. Trimethylamine N-oxide as a risk marker for ischemic stroke in patients with atrial fibrillation. J. Biochem. Mol. Toxicol. 2019;33(2) doi: 10.1002/jbt.22246. [DOI] [PubMed] [Google Scholar]
- 13.Büttner P.O.G., Hauke J., Holzwirth E., Obradovic D., Hindricks G., Thielea H., Kornej J. Trimethylamine N-oxide in atrial fibrillation progression. Int. J. Cardiol. Heart Vasc. 2020;29 doi: 10.1016/j.ijcha.2020.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gawalko M., Elliott A., Kadhim K., Sanders P., Linz D. A call for a more objective and longitudinal reporting of lifestyle components in cardiovascular research. Int. J. Cardiol. Heart Vasc. 2020;27 doi: 10.1016/j.ijcha.2020.100506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janeiro M.H., Ramirez M.J., Milagro F.I., Martinez J.A., Solas M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients. 2018;10(10) doi: 10.3390/nu10101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leeming E.R., Johnson A.J., Spector T.D., Le Roy C.I. Effect of diet on the gut microbiota: Rethinking intervention duration. Nutrients. 2019;11(2) doi: 10.3390/nu11122862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thaiss C.A., Itav S., Rothschild D., Meijer M.T., Levy M., Moresi C. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016;540(7634):544–551. doi: 10.1038/nature20796. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds A.C., Broussard J., Paterson J.L., Wright K.P., Jr., Ferguson S.A. Sleepy, circadian disrupted and sick: Could intestinal microbiota play an important role in shift worker health? Mol. Metab. 2017;6(1):12–13. doi: 10.1016/j.molmet.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schugar R.C., Shih D.M., Warrier M., Helsley R.N., Burrows A., Ferguson D. The TMAO-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the Beiging of white adipose tissue. Cell Rep. 2017;19(12):2451–2461. doi: 10.1016/j.celrep.2017.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Missailidis C., Hallqvist J., Qureshi A.R., Barany P., Heimburger O., Lindholm B. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]