Highlights
-
•
Different protocols and timing of surveillance exist for patients with BD-IPMN.
-
•
BD-IPMN are at risk of developing malignancy even during long surveillance.
-
•
In our study group incidence of pancreatic cancer in patients with BD-IPMN is 2.9%.
-
•
Worrisome features and high-risk stigmata developed in a low percentage of BD-IPMN.
Keywords: Branch duct - intraductal papillary mucinous neoplasms (BD-IPMN), Pancreatic cancer, Worrisome features, High-risk stigmata, Magnetic resonance cholangiopancreatography (MRCP)
Abstract
Purpose
To evaluate the outcome of a MR imaging procotol in assessing the evolution of individuals with branch duct - intraductal papillary mucinous neoplasms (BD-IPMN) without worrisome features (WF) and/or high risk stigmata (HRS) at the time of the diagnosis in a follow-up period of at least 10 years.
Material and methods
A retrospective revision of a prospectively collected radiological database including a total number of 600 patients who were investigated and diagnosed with “presumed” diagnosis of BD-IPMN at MRI/MRCP at our Department since 2008 was performed. Inclusion criteria were: 1) absence of worrisome features and/or high-risk stigmata at the time of diagnosis (baseline); 2) a radiological follow-up with abdominal MRI/MRCP of at least 10 years. Changes in cysts size, development of WF, HRS and pancreatic cancer, and any other modification during the follow-up were retrospectively analysed by two observers in consensus.
Results
Sixty-nine patients fulfilled all the inclusion criteria. During surveillance, the cysts remained dimensionally unchanged or slightly reduced in size in 26.2% and 4.3% of cases respectively, whereas cyst enlargement was demonstrated in 69.5% of cases. Median annual growth rate was of 0.97 ± 0.87 mm/yr (range 0.13-5.0). WF and HRS developed in 10/69 (14.5%) and 3/69 (4.3%) cases, respectively. The incidence of pancreatic cancer in patients with BD-IPMN was 2.9%.
Conclusion
Our data confirm the low risk of pancreatic cancer development in patients with BD-IPMN, thus justifying an imaging follow-up. Worrisome features and high-risk stigmata were promptly identified during the follow-up, supporting the utility of our surveillance MR imaging protocol.
1. Introduction
Intraductal papillary mucinous neoplasms (IPMNs) of pancreas are cystic tumors of mucin-producing cells that originate from main pancreatic duct or its branches.
IPMNs are most frequently identified in patients with age of 50-60 years [1] and occur most often in the pancreatic head and uncinate process [2].
Commonly, small intraductal tumors are occasional radiological findings at imaging performed for other reasons. In fact, they are usually asymptomatic and may remain silent for years. When symptomatic, clinical presentation can be aspecific. Patients may complain abdominal pain, weight loss, jaundice, new-onset diabetes and more frequently, episodes of pancreatitis [3,4].
IPMNs are commonly classified into three types based on radiological imaging findings and/or histology: main duct-IPMN (MD-IPMN), branch duct-IPMN (BD-IPMN), and mixed type [5].
MD-IPMN is defined by the presence of diffuse or segmental dilation of the main pancreatic duct (MPD) greater than 5 mm, without other causes of obstruction. However, according to recent reports in literature, a lower threshold for MPD dilation (5 mm) is suggested, which also leads to an increase of the sensitivity for radiologic diagnosis of MD-IPMN [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14]].
BD-IPMNs exclusively involve the secondary ducts. At imaging, they consist of single or more likely multiple, often multifocal, unilocular or septated cystic lesions in communication with the main pancreatic duct [5].
In mixed type IPMN, the criteria for both MD-IPMN and BD-IPMN are met [5]. Histologically, IPMNs may include hyperplastic lesions, various grades of dysplasia and carcinomas. In fact, IPMNs are at risk of degeneration and malignant transformation: it is therefore important to distinguish malignant from benign forms in order to schedule a proper follow-up and/or eventually plan surgical treatment.
Due to the high malignant potential of main-duct and mixed type IPMNs, many guidelines recommend surgical resection for these types of neoplasm at the time of diagnosis [5,[15], [16], [17]]. On the other hand, follow-up is indicated for patients with BD-IPMN in absence of any suspicious features at the time of the diagnosis, since during surveillance the reported incidence of pancreatic carcinogenesis is lower (3-8%) [[15], [16], [17], [18], [19], [20], [21]]. However, some major differences in timing and duration of follow-up across the studies exist [22].
Surgical resection is recommended when imaging or clinical features suggesting development of malignancy are observed during the surveillance period [22].
Therefore, the purpose of our study was to evaluate the outcome of a MR imaging procotol in assessing the evolution of individuals with BD-IPMN without worrisome features (WF) and/or high risk stigmata (HRS) at the time of the diagnosis with a follow-up of at least 10 years.
2. Material and methods
Ethical approval was waived by the local Ethics Committee of our hospital in view of the retrospective nature of the study and all the procedures being performed were part of the routine care. Before MRI examination, all patients provided written informed consent to the processing of personal data even for study purposes. Patients’ data anonymization was performed.
2.1. Study design and patients’ characteristics
A retrospective revision of a prospectively collected radiological database including a total number of 600 patients who were investigated and diagnosed with IPMN at our Department since 2008 was performed. The patients were subclassified according to main duct involvement (MD-IPMNs), branch-duct subtype (BD-IPMNs) and mixed type.
Patient’s demographics and radiological records were collected. Among them, a group of patients with “presumed” diagnosis of BD-IPMN at MRI/MRCP was selected. Inclusion criteria were the absence of WF and/or HRS at the time of diagnosis (baseline) and a radiological follow-up with abdominal MRI/MRCP for at least 10 years. Worrisome features were represented by: cyst size ≥ 3 cm; presence of contrast-enhancing thickened walls and/or of non contrast-enhancing mural nodules; main pancreatic duct (MPD) caliber of 5-9 mm; MPD abrupt interruption; cyst growth > 5 mm every 2 years. High-risk stigmata were considered the precence of contrast-enhancing mural nodule ≥ 5 mm and a main pancreatic duct caliber ≥ 10 mm.
Patients with missing data and/or without 10-years follow-up were excluded from the analysis.
2.2. MRI protocol
All MR examinations were performed on 1.5 T system (Signa Excite HDxt; GE Healthcare, Milwaukee, Wisconsin, USA).
Before starting the examination, scopolamine methyl-bromide was administered intramuscularly to avoid peristaltic artefacts.
The standard imaging protocol included T1-weighted breath-hold SPGR in-phase and out-of-phase axial sequences and T2-weighted axial sequences (both breath-hold, single-shot fast spin-echo and respiratory-triggered, fat-suppressed fast spin-echo) of the upper abdomen including the pancreatic gland. Then, MRCP was performed by respiratory-triggered, three-dimensional, heavily T2-weighted fast spin-echo (3D FRFSE) sequence and breath-hold, thick-slab, single-shot FSE T2-weighted sequences performed in the coronal and oblique-coronal projections. Diffusion weighted imaging (DWI) of the pancreatic region was performed using an axial respiratory-triggered spin-echo echo-planar sequence with multiple b values (300, 500, 700, 1000s/mm²) in all diffusion directions. At the baseline and if there was a suspicion of degeneration during the follow-up, a three-dimensional fat-suppressed Liver Acquisition with Volumetric Acceleration (LAVA) sequence was obtained in the axial and sometimes coronal plane before and after intravenous injection of Gadolinium-based contrast agents. Post-contrast images were obtained in the arterial, portal-venous and delayed (between 3 and 5 minutes) phases.
2.3. Follow-up protocol
According to the policy of our Regional Referral Center for Pancreatic Disease, patients were imaged with abdominal MRI/MRCP every 6 months in the first 2 years from diagnosis and then yearly in the absence of radiological signs of progression.
Changes in cysts size, development of WF, HRS and pancreatic cancer (PC), and any other modification during the follow-up were retrospectively analysed by two observers in consensus with experience of >15 years and 5 years in abdominal radiology, respectively.
In case of development of WF in the follow-up period, patients were investigated with endoscopic ultrasound (EUS). Surgery was indicated in case of mural nodules and/or solid components, malignancy at cytology and/or direct involvement of the MPD at EUS.
Surgical resection was also indicated in all patients who developed HRS during surveillance, if fit.
2.4. Statistical analysis
All statistical analysis was performed using SPSS software (version 25.0; IBM Corp., Armonk, NY, USA).
Categorical data were described by using absolute and relative frequency, while quantitative data were expressed as means ± standard deviation (SD). Chi square test was used to compare “surgery” (not; yes) with “diameter of Wirsung” (≤5 mm; >5 mm) and odds ratio was calculated. A value of P < 0.05 was considered statistically significant.
3. Results
Our final cohort included a series of sixty-nine patients fulfilling all the inclusion criteria.
During surveillance, the cysts remained dimensionally unchanged or slightly reduced in size in 18/69 (26.2%) and 3/69 (4.3%) cases, respectively. In the remaining 48/69 (69.5%) cases, cyst enlargement was demonstrated with median annual growth rate of 0.97 ± 0.87 mm/yr (range 0.13-5.0).
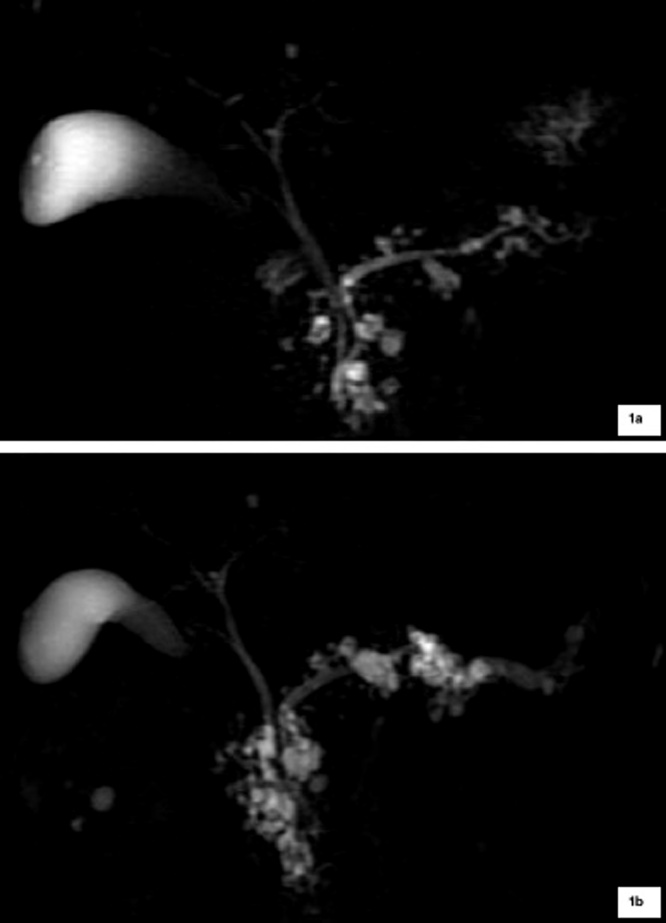
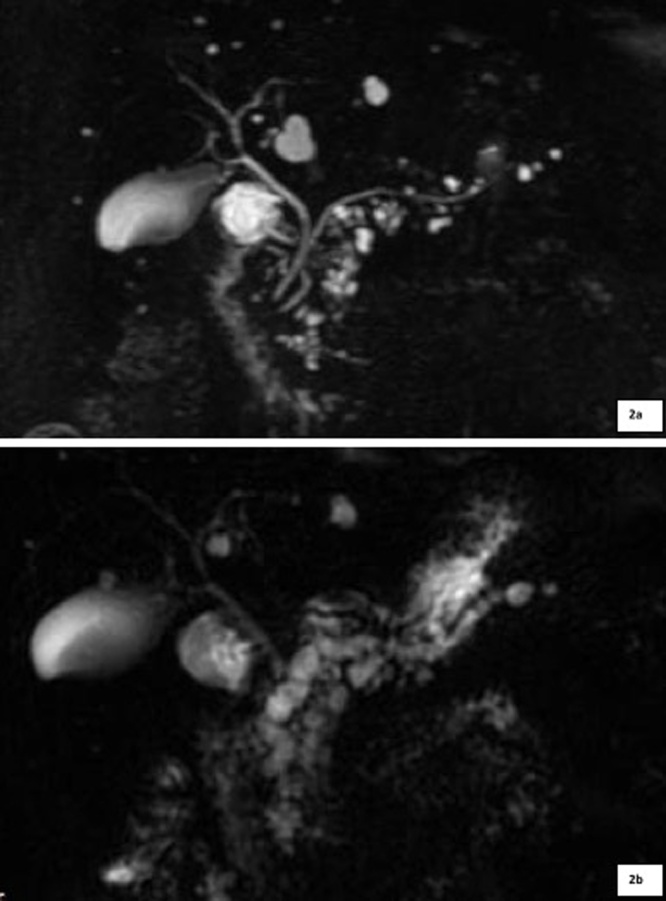
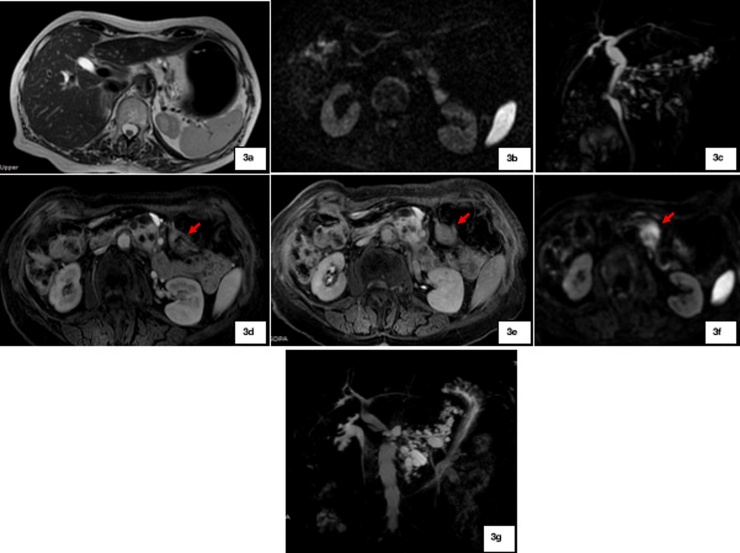
WF and HRS developed in 10/69 (14.5%) and 3/69 (4.3%) cases, respectively: WF included cyst dimension ≥ 3 cm (4/10), main pancreatic duct of 5-9 mm (3/10) [Fig. 1], cyst growth rate of 5 mm/2 yr (2/10) and non-enhancing mural nodule (1/10); HRS were represented by main pancreatic duct caliber greater than 10 mm [Fig. 2] in two patients, and presence of enhanced solid components in the other one [Fig. 3].
Fig. 1.
a: Patient with BD-IPMN without any WF or HRS and regular Wirsung caliber at baseline MRCP; 1b: After 8 years of follow-up, MRCP shows segmental dilation of Wirsung duct measuring 7 mm at the level of the pancreatic tail (WF), associated to a diffuse increase in cysts size.
Fig. 2.
a: Patient with BD-IPMN without any WF or HRS and regular Wirsung caliber at baseline MRCP; 2b: After 4 years of follow-up, MRCP well exhibits a diffuse dilation of the Wirsung duct with maximum diameter of 11 mm (HRS), associated to a diffuse increase in cysts size.
Fig. 3.
a-c: Patient with BD-IPMN without any WF or HRS at the baseline; 3d-g: After 8 years of follow-up, an enhanced solid lesion (red arrows) developed (3d-f) with restriction of diffusivity (3f) at the level of pancreatic body-tail. An increase in cysts size is also observed on MRCP (3 g). Patient underwent surgery with final histopathological diagnosis of PC.
In our series, all ten patients with WF were investigated with EUS and four of them did not show any signs of malignancy. The remaining 6 out of 10 patients with WF were candidate to surgery due to suspected signs of malignancy at EUS (mural nodules or solid components, malignancy at cytology and/or direct involvement of the MPD). Among them, only four out of six subjects underwent surgery since one patient refused surgical intervention (n = 1) and the other one was unfit (n = 1). In this subgroup, PC (n = 1) and IPMN with low grade dysplasia (n = 3) were the final histopathological diagnoses.
Among patients with HRS (n = 3), only one underwent surgery and PC was diagnosed at pathological examination. In the other two cases, surgery was not performed since one patient refused the surgical intervention (n = 1) and the other one was not eligible to the surgical procedure due to medical comorbidities (n = 1).
Overall, in our study group only five patients (7.2%) underwent surgery due to the development of HRS (n = 1) and WF (n = 4).
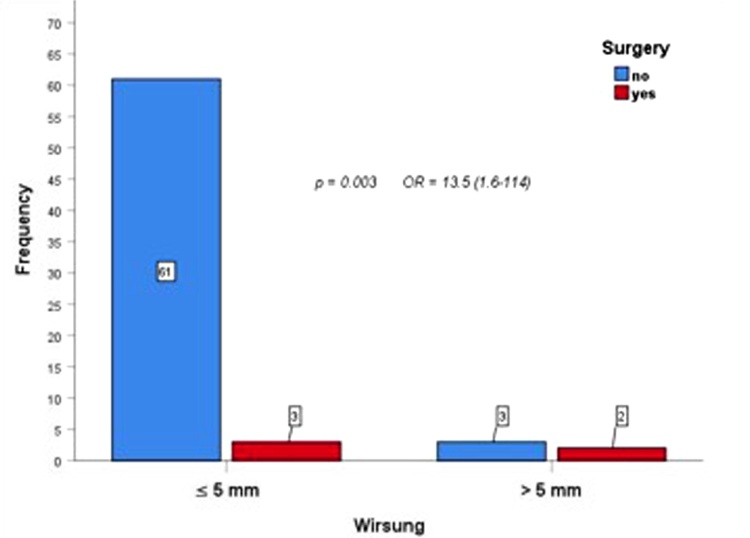
Furthermore, from the analysis of the caliber of the Wirsung duct in all 69 patients, a diameter less than or equal to 5 mm was associated with a significant decreased risk of surgical intervention, unlike the other group of patients with a Wirsung caliber greater than 5 mm (p = 0.003; odds ratio (OR) = 13.5) [Fig. 4].
Fig. 4.
The comparison between surgery and diameter of Wirsung duct showed that patients with Wirsung’s diameter less than or equal to 5 mm were associated with a significant decreased risk of surgical procedure, unlike the other group of patients with a Wirsung caliber greater than 5 mm (p = 0.003; odds ratio (OR) = 13.5)
4. Discussion
The term intraductal papillary mucinous tumor was adopted in 1997 [23]. Since then, there has been a rising interest in IPMNs because their diagnosis is constantly growing, especially for BD-IPMN, with increasing prevalence in the last years, which is mainly due to the improvement of radiological imaging modalities.
Several guidelines indicating how to follow-up patients with BD-IPMN are available, with different protocols and timing of surveillance programs.
The American Gastroenterological Association (AGA) guidelines indicate to stop follow-up of asymptomatic cysts of the pancreatic gland with no or minimal change during 5-year surveillance [15,24]. Otherwise, according to the “European evidence-based guidelines on pancreatic cystic neoplasms”, individuals with IPMN without indication for surgery at the time of diagnosis should be followed up until they are no longer fit for surgery [17]. Actually, there is a lack of long-term surveillance data about patient with BD-IPMN in large population studies [22].
In this scenario, there is a strong need to establish surveillance programs for patients with BD-IPMNs, due to their possible evolution into pancreatic ductal adenocarcinoma. In fact, while surgical resection is recommended at the time of diagnosis for main-duct IPMNs due to their high malignant potential [[15], [16], [17],20], individuals with BD-IPMN should be followed-up.
Our experience confirms that patients with BD-IPMN are at risk of developing pancreatic carcinoma even during long surveillance (2.9% of patients in our series). Furthermore, a recent study showed that there are no individuals with BD-IPMN with “zero risk” for cancer development [25]. In addition, even the timing of the follow-up is still matter of debate. According to the European evidence-based guidelines on pancreatic cystic neoplasms, a 6-month follow-up in the first year and yearly thereafter is adequate when there aren’t suspicious features that establish an indication for surgery. However, as mentioned before, stricter protocols may be used: in accordance to our internal policy developed by a multidisciplinary team (which included radiologists, oncologists and surgeons) and based on medical data reported in literature and local expertise, patients are imaged with abdominal MRI/MRCP every 6 months in the first 2 years from diagnosis and then each year in the absence of clinical/radiological signs of progression until unfit for surgery. In our experience, EUS should be utilized in all patients with WF in order to select candidates to surgery.
MRI is the adopted imaging modality for the follow-up of individuals with IPMN according to the European evidence-based guidelines on pancreatic cystic neoplasms (PCNs). We agree that MRI surveillance protocols are safe because only a minimal portion of BD-IPMNs requires surgical resection [25]. In this context, MRI is the best option for surveillance because of its accuracy, even if it is a time-consuming and expensive imaging modality. MR imaging protocol should always include the whole pancreas in the follow-up because of an increased risk of new-onset cancer in the entire glandular parenchyma. However, our MRI follow-up protocol is a shorter protocol since it includes the same sequences as the first examination except use of Gadolinium-enhanced T1-weighted sequences, that were utilized only if there was a suspicion of degeneration on non-enhanced study. For the surveillance of PCNs Pozzi-Mucelli RM et al [26] reported that short-protocol MRI provides information equivalent to the more time-consuming and costly comprehensive-protocol. According to their experience, evaluation of imaging risk factors in PCNs is comparable with both MRI-protocols. We agree with these observations, but we think that it is important to utilize diffusion-weighted imaging also in the short-protocol MRI since it is little time-consuming and can provide useful information in identifyng possible risk factors of BD-IPMN.
During the follow-up, we mainly focused on the development of WF e HRS; in addition, we evaluated also cyst growth rate because it has been reported as the most important predictor of malignancy and survival [25,27]. In fact, even though most BD-IPMNs do not progress during their natural history, a rapid increase in size during surveillance must not be underestimated because it may represent a predictor of PC development. In the present study, most cysts increased slightly in size during follow-up: 0.97 ± 0.87 mm/yr.
Another crucial point is the caliber of the MPD during the surveillance. In our study group, patients with a Wirsung duct caliber greater than 5 mm were associated with a significant increased risk of surgery. Therefore, when MPD diameter starts to increase during the follow-up, patients should be candidate to intensive surveillance and surgery should be considered.
In our series of 69 patients with BD-IPMNs without imaging risk factors at the time of diagnosis, 13 out of them (19%) developed WF and/or HRS during the follow-up. Our analysis confirms previous data: in fact, even if the risk of development PC is relatively minimal, it is still present longlife, both immediately after diagnosis and during long period of surveillance. Therefore, we support repeated observations until patients are considered unfit to surgery. We do not recommend follow-up discontinuation even in case of indolent cysts without radiological or clinical signs of progression.
The limitations of our study are: 1) the retrospective study design; 2) the lack of comparison with clinical and laboratory test results. However, our study was aimed to test the reliability of MR signs in the follow-up of patients with BD-IPMN; 3) the relatively small sample of patients with a long follow-up (more than 10 years). This allowed us to formulate only preliminary data about the progressive evolution of BD-IPMNs. In fact, considering the high prevalence of IPMN in the population [28], a larger number of patients with BD-IPMN with 10 years of follow-up should be evaluated to elucidate better the clinical course of patients with BD-IPMN and to compare the incidence of pancreatic carcinoma after 10 years follow-up with respect to the general population.
5. Conclusions
In our series of patients with BD-IPMN that were followed-up with MRI/MRCP for at least 10-years, the incidence of pancreatic cancer was 2.9%, thus justifying an imaging follow-up. Worrisome features and high-risk stigmata developed in 14.5% and 4.3% of patients, respectively and were promptly identified supporting the utility of our surveillance MR imaging protocol.
Future goals should include long-lasting follow-up in a large series of patients, with particular attention to rapidly changing BD-IPMNs. The evolution of the entity IPMN requires a deep knowlegde of the most recent guidelines and a continuous updating of them, in order to identify possible “new stigmata” of malignancy and define the best timing of imaging follow-up.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Piero Boraschi: Conceptualization, Methodology, Investigation, Project administration, Writing - review & editing. Gaia Tarantini: Conceptualization, Methodology, Data curation, Writing - original draft. Francescamaria Donati: Conceptualization, Methodology, Investigation, Visualization. Paola Scalise: Data curation, Writing - original draft. Rosa Cervelli: Data curation, Formal analysis, Visualization. Davide Caramella: Supervision, Validation.
Declaration of Competing Interest
The authors report no declarations of interest
References
- 1.Silas A.M., Morrin M.M., Raptopoulos V., Keogan M.T. Intraductal papillary mucinous tumors of the pancreas. AJR Am J Roentgenol. 2001;176(1):179–185. doi: 10.2214/ajr.176.1.1760179. [DOI] [PubMed] [Google Scholar]
- 2.Sugiyama M., Atomi Y. Intraductal papillary mucinous tumors of the pancreas: imaging studies and treatment strategies. Ann Surg. 1998;228(5):685–691. doi: 10.1097/00000658-199811000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Procacci C., Megibow A.J., Carbognin G., Guarise A., Spoto E., Biasiutti C., Pistolesi G.F. Intraductal papillary mucinous tumor of the pancreas: a pictorial essay. Radiographics. 1999;19(Nov-Dec (6)):1447–1463. doi: 10.1148/radiographics.19.6.g99no011447. [DOI] [PubMed] [Google Scholar]
- 4.Lubezky N., Ben-Haim M., Nakache R., Lahat G., Blachar A., Brazowski E., Santo E., Klausner J.M. Clinical presentation can predict disease course in patients with intraductal papillary mucinous neoplasm of the pancreas. World J Surg. 2010;34(Jan (1)):126–132. doi: 10.1007/s00268-009-0269-y. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M., Chari S., Adsay V., Fernández-del Castillo C., Falconi M., Shimizu M. International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6(1–2):17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 6.Serikawa M., Sasaki T., Fujimoto Y., Kuwahara K., Chayama K. Management of intraductal papillary-mucinous neoplasm of the pancreas: treatment strategy based on morphologic classification. J Clin Gastroenterol. 2006;40(Oct (9)):856–862. doi: 10.1097/01.mcg.0000225609.63975.6f. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt C.M., White P.B., Waters J.A., Yiannoutsos C.T., Cummings O.W., Baker M., Howard T.J., Zyromski N.J., Nakeeb A., DeWitt J.M., Akisik F.M., Sherman S., Pitt H.A., Lillemoe K.D. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246(Oct (4)):644–651. doi: 10.1097/SLA.0b013e318155a9e5. discussion 651-654. [DOI] [PubMed] [Google Scholar]
- 8.Nagai K., Doi R., Kida A., Kami K., Kawaguchi Y., Ito T., Sakurai T., Uemoto S. Intraductal papillary mucinous neoplasms of the pancreas: clinicopathologic characteristics and long-term follow-up after resection. World J Surg. 2008;32(Feb (2)):271–278. doi: 10.1007/s00268-007-9281-2. [DOI] [PubMed] [Google Scholar]
- 9.Hwang D.W., Jang J.Y., Lee S.E., Lim C.S., Lee K.U., Kim S.W. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg. 2012;397(Jan (1)):93–102. doi: 10.1007/s00423-010-0674-6. [DOI] [PubMed] [Google Scholar]
- 10.Mimura T., Masuda A., Matsumoto I., Shiomi H., Yoshida S., Sugimoto M., Sanuki T., Yoshida M., Fujita T., Kutsumi H., Ku Y., Azuma T. Predictors of malignant intraductal papillary mucinous neoplasm of the pancreas. J Clin Gastroenterol. 2010;44(Oct (9)):e224–9. doi: 10.1097/MCG.0b013e3181d8fb91. [DOI] [PubMed] [Google Scholar]
- 11.Bournet B., Kirzin S., Carrere N., Portier G., Otal P., Selves J., Musso C., Suc B., Moreau J., Fourtanier G., Pradère B., Lazorthes F., Escourrou J., Buscail L. Clinical fate of branch duct and mixed forms of intraductal papillary mucinous neoplasia of the pancreas. J Gastroenterol Hepatol. 2009;24(Jul (7)):1211–1217. doi: 10.1111/j.1440-1746.2009.05826.x. [DOI] [PubMed] [Google Scholar]
- 12.Waters J.A., Schmidt C.M., Pinchot J.W., White P.B., Cummings O.W., Pitt H.A., Sandrasegaran K., Akisik F., Howard T.J., Nakeeb A., Zyromski N.J., Lillemoe K.D. CT vs MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg. 2008;12(Jan (1)):101–109. doi: 10.1007/s11605-007-0367-9. [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto S., Lawler L.P., Horton K.M., Eng J., Hruban R.H., Fishman E.K. MDCT of intraductal papillary mucinous neoplasm of the pancreas: evaluation of features predictive of invasive carcinoma. AJR Am J Roentgenol. 2006;186(Mar (3)):687–695. doi: 10.2214/AJR.04.1820. [DOI] [PubMed] [Google Scholar]
- 14.Crippa S., Fernández-del Castillo C., Salvia R., Finkelstein D., Bassi C., Domingues I., Muzikansky A., Thayer S.P., Falconi M., Mino-Kenudson M., Capelli P., Lauwers G.Y., Partelli S., Pederzoli P., Warshaw A.L. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8(Feb (2)):213–219. doi: 10.1016/j.cgh.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vege S.S., Ziring B., Jain R., Moayyedi P. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148(Apr (4)):819–822. doi: 10.1053/j.gastro.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Stark A., Donahue T.R., Reber H.A., Hines O.J. Pancreatic Cyst Disease: A Review. JAMA. 2016;315(May (17)):1882–1893. doi: 10.1001/jama.2016.4690. [DOI] [PubMed] [Google Scholar]
- 17.European Study Group on Cystic Tumours of the Pancreas European evidence based guidelines on pancreatic cystic neoplasms. Gut. 2018;67(May (5)):789–804. doi: 10.1136/gutjnl-2018-316027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakubo K., Tada M., Isayama H., Sasahira N., Nakai Y., Yamamoto K., Kogure H., Sasaki T., Hirano K., Ijichi H., Tateishi K., Yoshida H., Koike K. Incidence of extrapancreatic malignancies in patients with intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60(Sep (9)):1249–1253. doi: 10.1136/gut.2010.227306. [DOI] [PubMed] [Google Scholar]
- 19.Kleeff J., Korc M., Apte M., La Vecchia C., Johnson C.D., Biankin A.V., Neale R.E., Tempero M., Tuveson D.A., Hruban R.H., Neoptolemos J.P. Pancreatic cancer. Nat Rev Dis Primers. 2016;(Apr (2)):16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M., Fernandez-Del Castillo C., Kamisawa T., Jang J.Y., Levy P., Ohtsuka T., Salvia R., Shimizu Y., Tada M., Wolfgang C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17(Sep - Oct (5)):738–753. doi: 10.1016/j.pan.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Nagata N., Kawazoe A., Mishima S., Wada T., Shimbo T., Sekine K., Watanabe K., Imbe K., Kojima Y., Kumazawa K., Mihara F., Tokuhara M., Edamoto Y., Igari T., Yanase M., Mizokami M., Akiyama J., Uemura N. Development of Pancreatic Cancer, Disease-specific Mortality, and All-Cause Mortality in Patients with Nonresected IPMNs: A Long-term Cohort Study. Radiology. 2016;278(Jan (1)):125–134. doi: 10.1148/radiol.2015150131. [DOI] [PubMed] [Google Scholar]
- 22.Oyama H., Tada M., Takagi K., Tateishi K., Hamada T., Nakai Y., Hakuta R., Ijichi H., Ishigaki K., Kanai S., Kogure H., Mizuno S., Saito K., Saito T., Sato T., Suzuki T., Takahara N., Morishita Y., Arita J., Hasegawa K., Tanaka M., Fukayama M., Koike K. Long-term Risk of Malignancy in Branch Duct Intraductal Papillary Mucinous Neoplasms. Gastroenterology. 2019 doi: 10.1053/j.gastro.2019.08.032. Aug 29. Gastroenterology. 2019 Aug 29. [DOI] [PubMed] [Google Scholar]
- 23.Solcia E., Capella C., Kloppel G. Atlas of tumor pathology, fasc 20, ser 3. Armed Forces Institute of Pathology; Washington, DC: 1997. Tumors of the pancreas; pp. 53–64. [Google Scholar]
- 24.Scheiman J.M., Hwang J.H., Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148(Apr (4)):824–848. doi: 10.1053/j.gastro.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Marchegiani G., Andrianello S., Pollini T., Caravati A., Biancotto M., Secchettin E., Bonamini D., Malleo G., Bassi C., Salvia R. "Trivial" Cysts Redefine the Risk of Cancer in Presumed Branch-Duct Intraductal Papillary Mucinous Neoplasms of the Pancreas: A Potential Target for Follow-Up Discontinuation? Am J Gastroenterol. 2019;114(Oct (10)):1678–1684. doi: 10.14309/ajg.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 26.Pozzi-Mucelli R.M., Rinta-Kiikka I., Wünsche K., Laukkarinen J., Labori K.J., Ånonsen K., Verbeke C., Del Chiaro M., Kartalis N. Pancreatic MRI for the surveillance of cystic neoplasms: comparison of a short with a comprehensive imaging protocol. Eur Radiol. 2017;27(Jan (1)):41–50. doi: 10.1007/s00330-016-4377-4. [DOI] [PubMed] [Google Scholar]
- 27.Pandey P., Pandey A., Luo Y., Aliyari Ghasabeh M., Khoshpouri P., Ameli S., O’Broin-Lennon A.M., Canto M., Hruban R.H., Goggins M.S., Wolfgang C., Kamel I.R. Follow-up of Incidentally Detected Pancreatic Cystic Neoplasms: Do Baseline MRI and CT Features Predict Cyst Growth? Radiology. 2019;292(Sep (3)):647–654. doi: 10.1148/radiol.2019181686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Chiaro M., Ateeb Z., Hansson M.R., Rangelova E., Segersvärd R., Kartalis N., Ansorge C., Löhr M.J., Arnelo U., Verbeke C. Survival Analysis and Risk for Progression of Intraductal Papillary Mucinous Neoplasia of the Pancreas (IPMN) Under Surveillance: A Single-Institution Experience. Ann Surg Oncol. 2017;24(Apr (4)):1120–1126. doi: 10.1245/s10434-016-5661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]