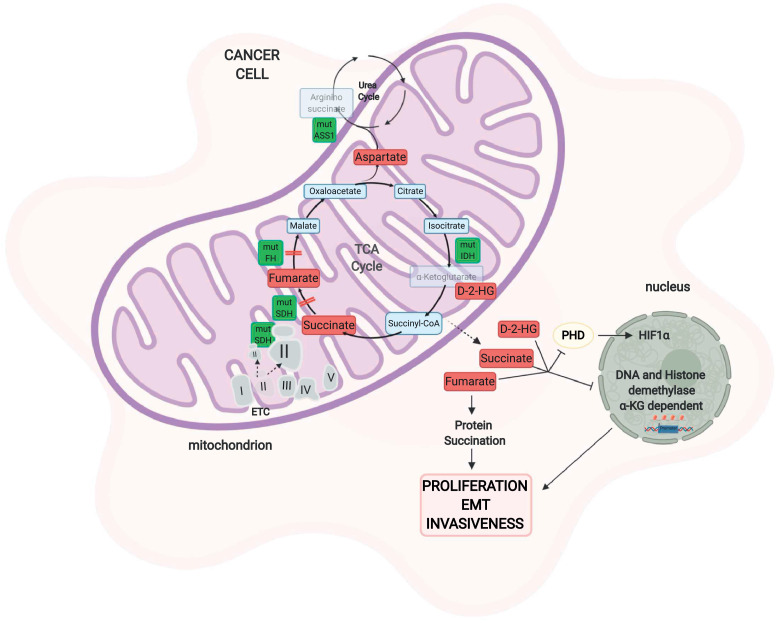
Fig. 3.
Mitochondrial enzyme mutations and cancer metabolism. Mutations in enzymes of the TCA cycle or other metabolic pathways as well as components of the electron transport chain alter the metabolome in response to altered mitochondrial metabolism. Mutations in SDH can lead to two different outcomes. The loss of SDH causes succinate accumulation that inhibits PHD, stabilizes HIF1, and inhibits α-KG-dependent histone and DNA demethylases, leading to the activation of proliferative pathways. On the other hand, SDH overexpression can sustain mitochondrial cancer metabolism due to the conversion of succinate into fumarate, which sustains the TCA cycle. Mutations in fumarate hydratase, which increase fumarate concentrations, lead to the inhibition of PHD and histone demethylases, promoting proliferation. Fumarate is also involved in a cysteine post-transcriptional modification, called succination. Isocitrate dehydrogenase (IDH) mutations in cancer help the generation of a neomorphic enzyme that converts isocitrate into 2-hydroxyglutarate (2-HG), a metabolite that exerts its oncogenic effect on PHD and epigenetic regulation. Epigenetic silencing of the urea cycle enzyme argininosuccinate synthase 1 (ASS1) leads to the accumulation of aspartate, which elicits tumorigenesis. SDH: succinate dehydrogenase, PHD: prolyl hydroxylase, HIF-1: hypoxia-inducible factor-1, IDH: isocitrate dehydrogenase, D-2-HG: D-2-hydroxyglutarate, ASS1: argininosuccinate synthase. "Created with BioRender.com."