Abstract
Malaria, leishmaniasis and trypanosomiasis are arthropod-borne, parasitic diseases that constitute a major global health problem. They are generally found in developing countries, where lack of access to preventive tools and treatment hinders their management. Because these parasites share an increased demand on glucose consumption with most cancer cells, six compounds used in anti-tumoral research were selected to be tested as antiparasitic agents in in vitro models of Leishmania infantum, Trypanosoma brucei, T. cruzi, and Plasmodium falciparum: dichloroacetic acid (DCA), 3-bromopyruvic acid (3BP), 2-deoxy-D-glucose (2DG), lonidamine (LND), metformin (MET), and sirolimus (SIR). No parasite-killing activity was found in L. infantum promastigotes, whereas DCA and 3BP reduced the burden of intra-macrophagic amastigotes. For T. brucei all selected compounds, but 2DG, decreased parasite survival. DCA, 2DG, LND and MET showed parasite-killing activity in T. cruzi. Finally, anti-plasmodial activity was found for DCA, 2DG, LND, MET and SIR. These results reinforce the hypothesis that drugs with proven efficacy in the treatment of cancer by interfering with ATP production, proliferation, and survival cell strategies might be useful in treating threatening parasitic diseases and provide new opportunities for their repurposing.
Keywords: Bionergetic modulators, Protozoan parasites, Repurposing, Sirolimus, Dichloroacetate, 3-bromopyruvate, 2-deoxy-D-glucose, Lonidamine, Metformin
Graphical abstract
Highlights
-
•
Parasitic diseases are prevalent among the poorest of the poor.
-
•
Some parasitic protists degrade glucose into CO2 even aerobically making this a target.
-
•
Degrading glucose into CO2 (Warburg effect) is also characteristic for cancer cells.
-
•
Repurposing cancer glycolysis blockers may provide cost-effective treatments for the poorest.
1. Introduction
Arthropod-borne parasitic infections are responsible for a number of severe diseases found around the world. They include primarily malaria, visceral leishmaniasis (VL), and American and African trypanosomiasis (Chagas disease and Human African Trypanosomiasis (HAT), also known as sleeping sickness, respectively). Malaria is caused by parasites belonging to the Plasmodium genus with P. falciparum being the most pathogenic for humans. VL, Chagas disease and HAT are brought about by Leishmania spp., Trypanosoma cruzi, and T. brucei, respectively, all belonging to the Trypanosomatidae family. These diseases are currently considered neglected because they mainly affect developing countries where there is a lack of access to tools and drugs for prevention and treatment. The existence of both wild and domestic reservoirs makes controlling the spread of these diseases even more difficult. Despite considerable efforts undertaken throughout the last decades, which have resulted in a marked decline in their incidence, parasite diseases remain a major public health problem. The latest World Health Organization (WHO) report shows that these parasites cause diseases in more than 200 million people annually. In 2018, there were an estimated 228 million cases of malaria worldwide, around 90,000 people were diagnosed with VL, almost 1000 with HAT, and roughly 7 million people were estimated to be chronically affected by Chagas disease. For the same year, more than 400,000 individuals, many of them children, died due to these parasitic infections (World Health Organization, 2020).
No vaccines have yet been commercialized for humans against these pathogens, thus the main approach for their management is based on pharmacological treatment. Developing new drugs involves both large budgets and long lead-times. In contrast, drug repurposing is a rapid and cheaper alternative because it employs validated molecular targets and drugs for new indications. This is especially helpful for neglected diseases since only a small percentage of total investment in health research goes to drug discovery for tropical diseases (Nwaka et al., 2009).
Because of their changing life conditions, these pathogens exhibit different metabolic profiles during their biological cycle, which are associated with major changes in central carbon metabolism. Of special interest is the energetic metabolism that these parasites exhibit within the host where they undergo rapid multiplication.
Bloodstream stages of T. brucei show the simplest metabolism of trypanosomatids, where the sole source of ATP is glucose via glycolysis, being pyruvate the main end product (Coley et al., 2011). T. cruzi and Leishmania spp amastigotes can degrade other metabolites than glucose, such as amino acids, and glycolysis is extended to a reversible succinate production pathway within the glycosome (Besteiro et al., 2002; Maugeri et al., 2011). The resulting pyruvate is transformed into alanine or transported to the mitochondrion, instead of being excreted to the external milieu. These parasites show a much more complex mitochondrial metabolism that is strongly connected to anabolic processes and OXPHOS, with a cytochrome-containing respiratory chain (Tielens and van Hellemond, 2009). Notwithstanding, the high metabolic demand to support rapid multiplication in the mammalian host, turns glycolysis into an essential process for these amastigote-stage trypanosomatids (Verlinde et al., 2001; Subramanian et al., 2015; Shah-Simpson et al., 2017), with special interest for NADPH regeneration and for the correct functioning of their PPP (Maugeri et al., 2003, 2011). As it is the case for trypanosomatids, P. falciparum intraerythrocytic asexual forms are highly dependent on glucose (Sherman, 1998), and preliminary results indicate that these parasites are aerobic glycolytic organisms with a modified TCA mainly fueled by glutamine and glutamate and low OXPHOS (Salcedo-Sora et al., 2014). Same as T. brucei bloodstream stage, P. falciparum glycolysis products are pyruvate and alanine. However, P. falciparum has a functional lactate dehydrogenase which transforms some of the generated pyruvate into lactate that is excreted to the external milieu (Salcedo-Sora et al., 2014; Penkler et al., 2015). As a result, glycolysis appears to be a particularly suitable target to be blocked, as these parasites tend to be more dependent on this pathway than the quiescent cells from differentiated tissues of their hosts (Renslo and McKerrow, 2006).
Interestingly, an increased glucose consumption and glycolytic activity is also a common feature in some studied disease models, as it is the case of cancer. Most cancer cell types are known to have an enhanced and modified glycolytic metabolism, even in the presence of oxygen (aerobic glycolysis), resulting in glucose transformation to lactate (Warburg et al., 1924; Warburg, 1956). In addition, there is a reduced flux of carbon to the mitochondrion which is highly involved in anabolic processes (Vander Heiden et al., 2009). Hence, glycolysis turns into a therapeutic target for proliferation-committed cells.
An imbalance in ATP production —either due to glucose deprivation, hypoxia, pharmacological disruptors, such as glycolysis inhibitors, or bioenergetic modulators— leads to activation of AMP-activated protein kinase (AMPK), an upstream protein of the mechanistic target of rapamycin (mTOR) (Hardie, 2011). As in the case of glycolysis, the mTOR route could be also a main therapeutic target for proliferation-committed cells, since it acts as a key homeostatic sensor that integrates microenvironmental and proliferation signals with bioenergetic metabolism to regulate biomass production and cell survival (Sabatini and Laplante, 2012). Furthermore, the mTOR route is strongly related to glycolysis as it is able to regulate the expression of many glucose transporters and some glycolysis enzymes through the Hypoxia-inducible Factor 1 (HIF-1), thus favoring aerobic glycolysis over mitochondrial respiration (Düvel et al., 2010).
These bioenergetics pathways have been proposed as potential and interesting targets for fighting protozoan diseases (Verlinde et al., 2001; Jacobs et al., 2011; Saldivia et al., 2013). Likewise, some energy modulators are already in use in cancer diagnosis, or in advanced phases of clinical trials (Scatena et al., 2008; Granchi and Minutolo, 2013), thus facilitating repurposing.
The aim of the present study was to test the hypothesis that drugs acting on these metabolic pathways, and whose efficacy in cancer research has been demonstrated, could be useful in the management of a number of parasitic diseases, including Chagas disease, HAT, VL and malaria. The following compounds were selected: 3-bromopyruvic acid (3BP), 2-deoxy-D-glucose (2DG), lonidamine (LND), dichloroacetic acid (DCA), metformin (MET), and sirolimus (SIR) (Table 1). LND, 3BP, and 2DG block early stages of the glycolytic route as they interfere with the activity of hexokinase (HK) or cannot be further metabolized (Paggi et al., 1988). Initially, 3BP was described as inhibiting HKII activity (Ko et al., 2001), however, it has been shown to also exhibit inhibitory activity on GAPDH, another enzyme of the same pathway (Ganapathy-Kanniappan et al., 2009). DCA and the biguanide MET were chosen because of their ability to modulate ATP and biomass production pathways at mitochondrial level. DCA switches cell metabolism from aerobic glycolysis to oxidative phosphorylation by restoring mitochondrial activity (Michelakis et al., 2008). MET blocks the mitochondrial respiratory chain complex I thus provoking unbalanced ATP production (Owen et al., 2000) and AMPK activation (Fryer et al., 2002). Finally, SIR was included because of its direct inhibiting activity on mTOR (Mita et al., 2003).
Table 1.
Selected drugs and their energetic and biomass production-related targets.
| Compound | Energy and biomass production-related target |
|---|---|
| 3-Bromopyruvic acid | GAPDH (Ganapathy-Kanniappan et al., 2009); Hexokinase II (Ko et al., 2001) |
| 2-Deoxy-D-glucose | Glycolysis (Nirenberg and Hogg, 1958) |
| Lonidamine | Hexokinase I (Paggi et al., 1988) |
| Sodium dichloroacetate | PDK (Michelakis et al., 2008) |
| Metformin | Complex I of the OXPHOS (Owen et al., 2000); AMP-activated protein kinase (Fryer et al., 2002) |
| Sirolimus | mTOR (Mita et al., 2003) |
Acronyms: GAPDH (glyceraldehyde-3-phosphate dehydrogenase), PDK (pyruvate dehydrogenase kinase), OXPHOS (oxidative phosphorylation), AMP (adenosine monophosphate), and mTOR (mechanistic target of rapamycin).
2. Material and methods
To initially assess the aptitude of the known compounds 2-deoxy-D-Glucose, 3-bromopyruvic acid, lonidamine, sirolimus, dichloroacetic acid and metformin to manage malaria, visceral leishmaniasis, and African and American trypanosomiasis, we first studied their associated in vitro effect. For that purpose, we tested the cytotoxicity of these compounds to host cells of each in vitro culture model based on cancer research literature, determining the range of possible therapeutic concentrations. Once established, these compounds were assayed on in vitro cultures of L. infantum promastigotes, intra-macrophagic L. infantum amastigotes, intra-cellular T. cruzi amastigotes, bloodstream T. brucei trypomastigotes, and P. falciparum-infected red blood cells. Parasites were co-cultured for 48–72 h with the studied compounds and their efficacy was recorded as the ability of each one to reduce the number of parasite forms. IC50 values were calculated and compared between models and with first-line drugs. Each test was performed three times.
2.1. Parasites and mammalian cell cultures
L. infantum JPC strain (MCAN/ES/1998/LLM-724) promastigotes were cultured at 26 °C in R15 medium [RPMI 1640 medium (Gibco®) supplemented with 15% heat-inactivated fetal bovine serum (FBS) (Gibco®), 2% HEPES 1M (Gibco®), 1% 10000 U/mL penicillin, and 10,000 μg/mL streptomycin (Gibco®)]. Weekly passages were performed. Metacyclic promastigotes for in vitro infections were obtained from a 6-day-old stationary culture. U937 human macrophages derived from a Caucasian histiocytic lymphoma were obtained from the European Collection of Cell Cultures (ECACC). Cells were cultured in RU937 media (RPMI-1640 medium supplemented with 10% FBS and 1% Penicillin/Streptomycin), and kept in a humid atmosphere at 37 °C and 5% CO2. Before carrying out each experiment, 1 × 106 U937 cells were seeded in a 25 mm2 culture flask and differentiated in a monolayer by adding Phorbol 12-myristate 13-acetate (PMA) at a final concentration of 50 nM for 48 h and then washed twice with 1 × phosphate buffered saline (1 × PBS), and fresh media added (Maia et al., 2007).
P. falciparum 3D7 (obtained from MR4-ATCC) chloroquine-sensitive parasites were cultured with human red blood cells (RBCs) obtained from the Blood and Tissue Bank (Catalonia, Spain), after approval from Hospital Clínic of Barcelona's Clinical Research Ethics Committee. Parasites were co-cultured with B + RBCs (3% hematocrit) in RPMI medium with 10% human AB + type serum, and kept at 37 °C in an atmosphere of 93% N2, 2% O2, and 5% CO2. In order to select ring stage parasites, a sorbitol synchronization protocol was followed, keeping infected erythrocytes at 37 °C for 10 min with 10 times its volume of sorbitol 5% (Trager and Jensen, 1976).
The T. brucei brucei 427 strain was maintained in HMI-9 culture medium [Iscove's modification of DMEM (IMDM; Cell Gro) supplemented with 10% FBS, 10%, Serum plus (SAFC), 0.05 mM Bathocuproinesulfonate, 1.5 mM L-cysteine, 1 mM hypoxanthine, 0.2 mM β-mercaptoethanol, 0.16 mM thymidine 1 mM pyruvate)] and passages were performed twice weekly.
CL strain T. cruzi overexpressing a tdTomato red fluorescent protein was used (Canavaci et al., 2010). Parasites were maintained in Vero cell cultures through weekly passage. Fibroblast-like kidney cells from African green monkey (Vero cells, ECACC) were purchased from Sigma Aldrich (Ref 84113001). Vero cells were maintained in culture media [RPMI 1640 supplemented with 10% fetal bovine serum, gentamycin (25 μg/mL), Penicillin/Streptomycin (0.1 mg/mL), L-glutamine (2 mM), sodium pyruvate 1 mM), and 2-mercaptoethanol (50 μM)] at 37 °C and 5% CO2. Passages were performed twice weekly. After 4–5 days of culture, trypomastigotes released from Vero cells were collected to carry out the drug screening assay.
2.2. Drugs
3BP (CAS N1113-59-3), DCA (CAS N 79-43-6), 2DG (CAS N 154-17-6), LND (CAS N 50264-69-2), MET (CAS N 1115-70-4), and SIR (CAS N 53123-88-9) (Table 1) were purchased from Sigma-Aldrich and freshly prepared before use in the appropriate culture media. The following reference drugs were selected as positive controls: chloroquine (CQ) for P. falciparum, benznidazole (BZD) for T. cruzi, meglumine antimoniate (Glucantime®, Sanofi-Avensis) for L. infantum intracellular amastigotes, amphotericyn B (AmB) (Sigma-Aldrich) for L. infantum promastigotes, and suramin (SUR) for T. brucei in vitro experiments.
2.3. Cytotoxicity to mammalian cells
In order to test toxicity on human macrophages, 5 × 104 U937 differentiated cells per well were seeded in 96-well microplates, and cultured with increasing concentrations of 3BP, DCA, 2DG, MET, LND, and SIR diluted in RU937 culture media. Each concentration was assayed in triplicate. The cells were kept at 37 °C in a 5% CO2 atmosphere. Cell viability was determined 48 h later by the MTT assay. Briefly, Thiazolyl Blue Tetrazolium Bromide (MTT) was added to each well at a final concentration of 0.5 mg/mL. After 4 h of incubation in the dark, 100 μL of HCl 0.1 N in isopropanol +10% Triton X-100 was added to each well to solubilize the resulting formazan crystals, and then read at a wavelength of 570 nm (reference 690 nm). The Alamar Blue (Invitrogen) assay was performed in the case of 3BP, in order to avoid interferences (Ganapathy-Kanniappan et al., 2010). Alamar Blue was added in an amount equal to 10% of the culture volume and plates were kept in the incubator for 24 h. Absorbance was read at 570 nm and 600 nm, and results were calculated following the manufacturer's recommendations.
For cytotoxicity assays on Vero cells, 1.7 × 104 cells were seeded in black, clear-bottom 96-well plates and incubated overnight at 37 °C and in 5% CO2. Serial dilutions of the drugs were added and incubated at 37 °C and in 5% CO2 for 72 h. Alamar Blue (AbD Serotec) was then added and the measurements performed 6 h later as described above.
In order to evaluate the compatibility of the selected drugs and erythrocytes, hemolysis was studied in vitro by direct spectrophotometry. RBCs (3% hematocrit) were cultured in round-bottom 96-well plates and incubated for 48 h with serial dilutions of the studied compounds in a humid atmosphere with concentrations of 5% CO2, 2% O2, and 93% N2. Each sample was assayed in duplicate, and a positive control for hemolysis was included by adding autoclaved distilled water to a well containing RBCs. The Harboe method was used to assess haemolysis (Harboe, 1959).
2.4. Effect of bioenergetic modulators on L. infantum
The killing effect of these drugs was assessed in vitro against the two stages of the biological cycle of Leishmania: motile promastigotes in the sand-fly vector and intracellular amastigotes in the host.
2.4.1. Anti-L. infantum promastigotes drug test
Promastigotes from a 4-day culture were seeded at 1 × 105 parasites per well in a 96-well microplate with increasing concentrations of drugs for 48 h at 26 °C. AmB at 0.25 μM was used as positive control. In order to determine parasite viability, the phosphatase activity assay was performed. Briefly, parasites were completely lysed using a solution made of 1% Triton X-100 and 2.9% sodium citrate in distilled water. Then, 5 mM of p-nitrophenyl phosphate was added to each well. In viable cells, p-nitrophenyl phosphate was hydrolyzed by intracellular acid phosphatases to p-nitrophenol. After 2 h of incubation at 37 °C, the reaction was stopped by adding 60 μL of NaOH 1 N to each well. Data were collected by reading the optic density at a wavelength of 405 nm (Yang et al., 1996).
2.4.2. Drug test on L. infantum intra-macrophage amastigotes
Cell line U397 was cultured in 25 mm2 flasks and differentiated as explained above. Metacyclic promastigotes (6 days’ culture) were added to the pre-washed differentiated cell culture at a parasite:cell ratio of 10:1, and co-cultured for 24 h. They were then washed with 1 × PBS to discard non-internalized promastigotes. Infected macrophages were re-suspended in RU937 and incubated in 8 well chamber slides at a density of 5 × 104 with increasing, but non-toxic to human macrophages, concentrations of 3BP, DCA, 2DG, SIR, LND, and MET for 48 h. Meglumine antimoniate at a concentration of 273 μM was used as positive control. Each concentration was tested in duplicate. Preparations were fixed with methanol and stained with Giemsa 11%. The numbers of infected macrophages and the intracellular parasites were recorded by direct microscopic count of 200 cells per sample.
2.5. P. falciparum growth inhibition assay
RBCs were cultured in RPMI and spiked P. falciparum ring infected erythrocytes to a final hematocrit of 3% and 0.8% infected blood cells. Cultures were then transferred to 96 microwell plates and exposed to increasing concentrations of 3BP, DCA, 2DG, MET, LND, and SIR prepared in media culture. CQ (80 nM) was used as reference drug to achieve parasite growth inhibition values above 90% (>IC90). Each concentration was assayed in triplicate and kept at 37 °C in a 5% CO2, 93% N2, and 2% O2 atmosphere. After 48 h, parasitemia was determined by fluorescence-assisted cell sorting [FACS] (BD LSRFortessa™ cell analyzer, Becton Dickinson), after staining infected RBCs with SYTO 11 (Molecular Probes, Life Technologies). Non-infected RBCs and infected non-treated RBCs were used as controls.
2.6. Screening of drugs against T. brucei
Cultured parasites were collected and spun for 10 min at 900 g to eliminate supernatant and were then cultivated at 5 × 105 parasites per well in a 96-well white, sterile plate in warm medium with serial dilutions of the selected compounds. Suramin (SUR) at 100 μM was used as positive control. Each concentration was tested in triplicate. After 24 h, Alamar Blue (Sigma) was added as previously described, and plates were incubated at 37 °C for 4 h before fluorescence was read at excitation (Ex) 530 nm and 590 nm emission (Em) wavelength.
2.7. Drug test on intracellular amastigotes of T. cruzi
Gamma-irradiated (2200 rad) Vero cells were plated in 96-well, black, clear-bottom plates at 3.6 × 104 cells per well and left overnight at 37 °C, in 5% CO2 atmosphere. Vero cells were then infected with 3.6 × 105 trypomastigotes of the CL tdTomato strain of T. cruzi per well for 5 h at 37 °C and 5% of CO2. After infection, the plates were washed once with Hanks’ solution to eliminate extracellular parasites and cultured again in the presence of serial dilutions of the selected compounds. Benznidazole was used as a positive control drug at a concentration of 2 μM. Each concentration was assayed in quadruplicate. After 72 h, fluorescence was measured (Ex = 544 nm; Em = 612 nm).
2.8. Data analysis
In all cases, results were expressed as the percentage inhibition of parasite growth in relation to the infected but non-treated controls for each assay (100 − [(mean parasitemia treated/mean parasitemia control) × 100]). In case of Plasmodium viability assays, values were firstly standardized based on that obtained with CQ for eliminating technique-dependent variability (post-treatment presence of DNA debris in samples with death parasites).
IC50 values were determined by using a sigmoidal dose-response with variable slope equation (Y = Bottom + ((Top - Bottom)/(1 + 10 (LogIC50 - X) - Hill Slope))) (GraphPad Prism Software v5).
3. Results
3.1. Leishmanicidal activity of bioenergetic modulators on L. infantum promastigotes and intramacrophagic amastigotes
The activity of DCA, 3BP, 2DG, LND, MET, and SIR on Leishmania promastigotes was assessed after culturing parasites with the drugs for 48 h. Only 3BP and 2DG showed a slight parasite-killing effect at doses of 37 μM and 0.2 mM, respectively, and did not reach the values obtained by the control drug AmB (0.25 μM) (36% survival rate) (data not shown). SIR, DCA, MET, and LND did not detectably impair L. infantum promastigote survival.
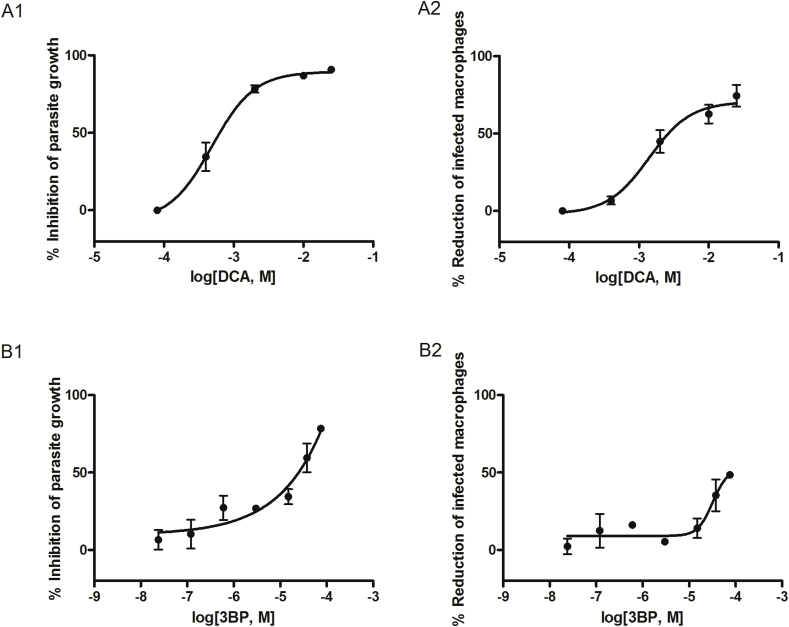
When these compounds were tested in an infection assay of L. infantum intra-macrophage amastigotes, an inhibitory, dose-dependent effect was observed in infected and treated cells with 3BP and DCA. The percentages of infected macrophages after 48 h of treatment with the highest, but non-cytotoxic doses of these compounds, were reduced by 74.4% for 25 mM of DCA and 48.4% for 75 μM of 3BP. Concurrently, the number of intracellular amastigotes decreased by 90.7% for DCA and 78.3% for 3BP, values 31.1% and 18.6% higher than those obtained with meglumine antimoniate at 273 μM, respectively (Fig. 1). Their parasite-killing activity is translated to IC50 values of 631.5 μM for DCA and 24.94 μM for 3BP (Table 2). The other tested drugs only showed a slight effect, and their anti-parasitic activity was lower than that produced by meglumine antimoniate (data not shown). SIR did not show any parasiticidal effect although a modest increase of the parasite burden and number of infected macrophages were observed at a concentration of 10 μM (data not shown).
Fig. 1.
Efficacy of DCA and 3BP on L. infantum-infected macrophages. Dose-response curves showing the inhibition of L. infantum intracellular amastigotes growth (1) and L. infantum-infected macrophages (2). The percentage inhibition was determined by comparing with untreated controls after 48 h of treatment with (A) DCA and (B) 3BP. Each point represents the mean ± standard error of the mean (SEM) obtained from three independent tests where each concentration was tested in duplicate.
Table 2.
IC50 values obtained from selected drugs during protozoan parasites in vitro studies.
| COMPOUND |
IN VITRO PARASITE MODEL |
Range of tested concentrations | ||||
|---|---|---|---|---|---|---|
|
P. falciparum Intraerythrocytic stage |
T. cruzi intracelular amastigotes |
T. brucei Bloodstream stage |
L. infantum intracelular amastigotes |
L. infantum infected macrophages |
||
| IC50 | IC50 | IC50 | IC50 | IC50 | ||
| 3BP | – | – | 7.66 ± 0.22 × 10−5 | 2.49 ± 0.23 × 10−5 | >7.50 × 10−5 | 7.50 × 10−5 to 2.40 × 10−8 |
| DCA | 5.39 ± 0.67 × 10−3 | 2.58 ± 0.59 × 10−2 | 1.24 ± 0.05 × 10−3 | 6.32 ± 1.63 × 10−4 | 2.69 ± 1.58 × 10−3 | 2.50 × 10−2 to 1.00 × 10−4 |
| 2DG | 4.19 ± 0.21 × 10−3 | 7.27 ± 0.47 × 10−3 | – | – | – | 1.60 × 10−2 to 2.00 × 10−3 |
| LND | 2.09 ± 0.13 × 10−4 | 8.24 ± 0.15 × 10−5 | 2.68 ± 0.26 × 10−5 | – | – | 3.00 × 10−4 to 1.2 × 10−6 |
| MET | 1.32 ± 0.18 × 10−3 | 1.85 ± 0.18 × 10−2 | 1.73 ± 0.15 × 10−2 | – | – | 4.00 × 10−2 to 6.4 × 10−6 |
| SIR | 2.50 ± 0.47 × 10−6 | – | 2.14 ± 0.11 × 10−6 | – | – | 1.00 × 10−5 to 0.10 × 10−6 |
Abbreviations: IC50 values are given in molar (M) ± standard deviation. Acronyms: 3BP (3-bromopyruvic acid), DCA (dichloroacetic acid), 2DG (2-deoxy-D-glucose), LND (lonidamine), MET (metformin), and SIR (sirolimus).
3.2. Antiplasmodial activity of bioenergetic modulators
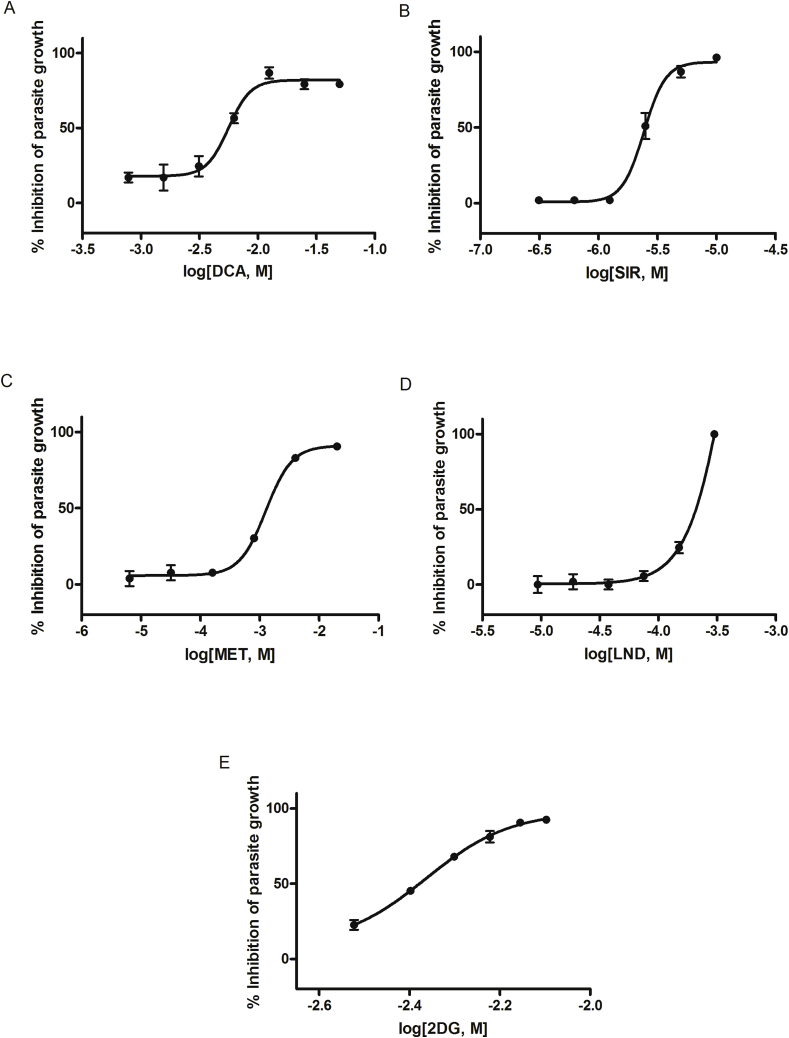
P. falciparum intraerythrocytic stages were sensitive in a dose-dependent manner to DCA, 2DG, SIR, and MET and reached growth inhibition values close to those obtained by CQ. IC50 values (Table 2) indicate P. falciparum is highly susceptible to SIR (IC50 = 2.50 μM) while other assayed compounds as DCA (IC50 = 5.39 mM), 2DG (IC50 = 4.19 mM), and MET (IC50 = 1.32 mM) needed higher concentrations to inhibit parasite growth. A slight effect on parasite growth inhibition was observed at high concentrations for 3BP. LND showed a peak of antimalarial activity at 300 μM (IC50 = 209.13 μM) (Fig. 2).
Fig. 2.
Growth inhibition of P. falciparum. Dose-dependent effect of (A) dichloroacetic acid, (B) sirolimus, (C) metformin, (D) lonidamine, and (E) 2-deoxy-D-glucose on P. falciparum in vitro assays. Data was obtained by flow cytometry and the inhibition of parasite growth was standardized with CQ-treated results and then calculated with respect to infected but untreated RBCs. Each concentration was tested in triplicate and data represent mean ± SEM of three independent experiments.
3.3. Anti-T. brucei activity of bioenergetic modulators
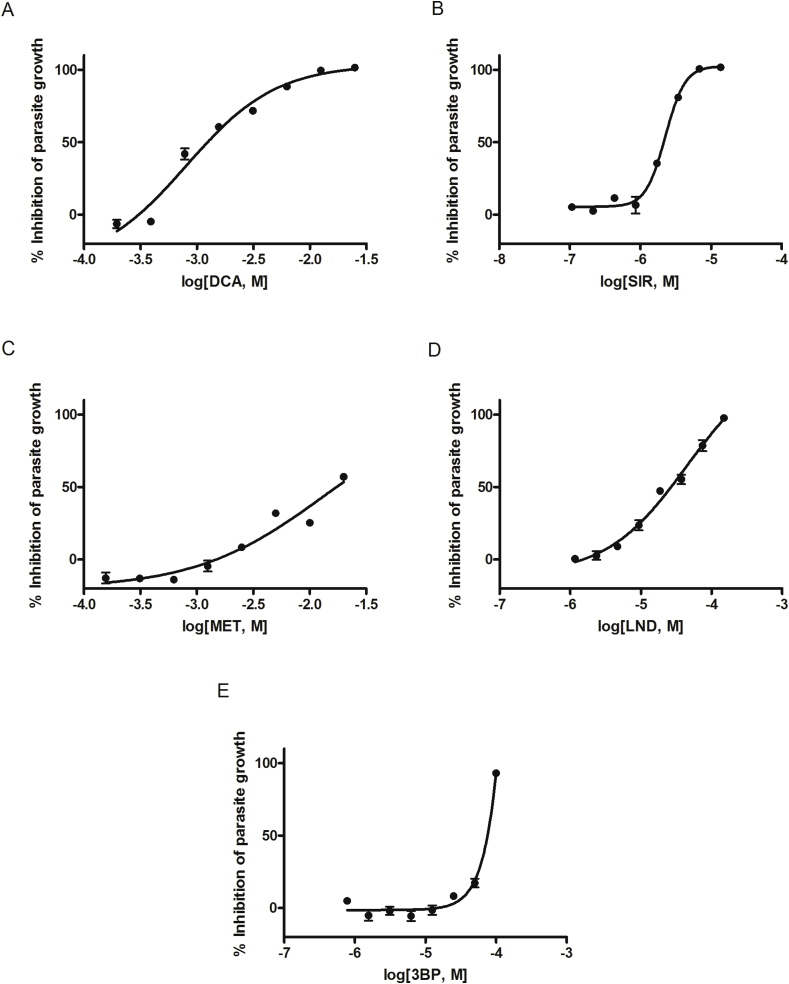
DCA and SIR achieved complete clearance of cultured T. brucei brucei parasites at 12.5 mM (IC50 = 1.24 mM) and 6.85 μM (IC50 = 2.14 μM) concentrations, respectively. Similar values were also obtained for LND (IC50 = 26.76 μM) and 3BP (IC50 = 76.57 μM), showing 97.7% and 93.2% parasite-killing activity, respectively. MET only produced 57% parasite elimination at 20 mM (IC50 = 17.30 mM) (Table 2). No parasite-killing activity was detected for 2DG. SUR 100 μM was used as a control drug, inducing nearly complete parasite clearance (96%) (Fig. 3).
Fig. 3.
Dose-response curves of anti-trypanosomal activity on T. brucei cultured parasites. Fluorescence values, obtained after culturing parasites with (A) dichloroacetic acid, (B) sirolimus, (C) metformin, (D) lonidamine, and (E) 3-bromopyruvic acid, were compared to non-treated controls to indicate their T. brucei-killing activity. Suramin was used as a positive control. Each point represents the mean ± standard error of the mean (SEM) obtained from three independent tests where each concentration was tested in triplicate.
3.4. Effect of bioenergetic modulators on T. cruzi amastigotes
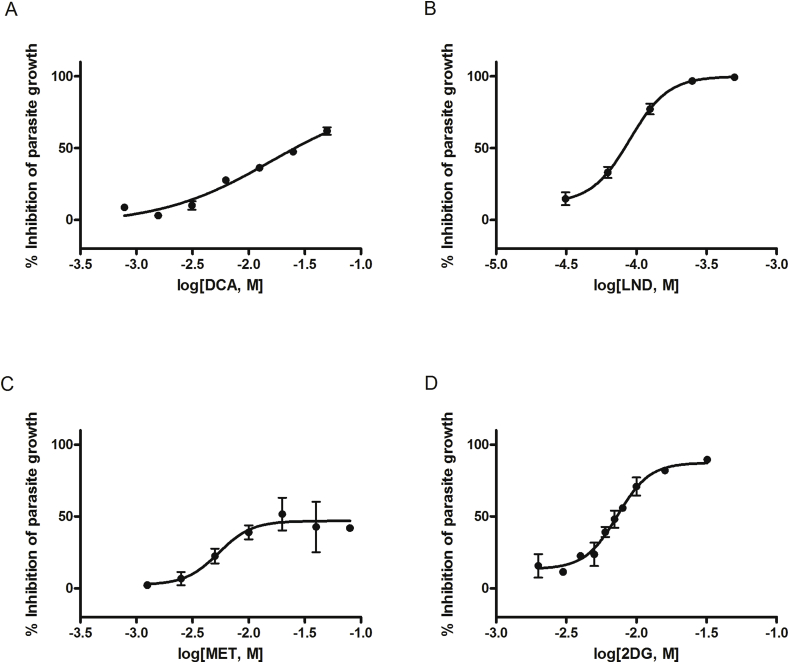
LND and 2DG produced the highest reduction in T. cruzi amastigote numbers, reaching inhibition values of 99.4% (IC50 = 82.41 μM) and 89.3% (IC50 = 7.27 mM), respectively. DCA and MET also decreased parasite survival but did not reach the level of the positive control. DCA action required high concentrations (up to 100 mM) in order to reach the highest value of 74.1% (IC50 = 25.82 mM), while MET only produced mild inhibition with a maximum of 51.7% at 20 mM (IC50 = 18.48 mM) (Table 2). A sustained mild reduction in fluorescence was detected at higher concentrations, which may suggest a trypanosomastatic effect (Fig. 4). In contrast, 3BP and SIR did not show consistent antitrypanosomal activity (data not shown).
Fig. 4.
Parasite-killing activity on T. cruzi intracellular amastigotes. The dose-dependent reduction in emitted fluorescence with respect to non-treated controls represents the parasite-killing power of (A) dichloroacetic acid, (B) lonidamine, (C) metformin, and (D) 2-deoxy-D-glucose. Each concentration was assayed in quadruplicate in three independent tests and mean values and SEM are represented in the figure.
4. Discussion
The aim of this study was to demonstrate and compare the in vitro effectiveness of different bioenergetic modulators previously used in diagnostic and anticancer studies on L. infantum, P. falciparum, T. cruzi, and T. brucei. These drugs were selected because of their modulating activity on enzymatic pathways known to be related to ATP production, proliferation, and cell survival strategies. To the best of our knowledge, this is the first time that some of these bioenergetic modulators have been specifically used for this purpose. DCA has demonstrated a remarkable in vitro parasiticidal activity in models of highly replicative stages of L. infantum, P. falciparum, T. cruzi, and T. brucei. Moreover, although 2DG has been utilized several times to measure glucose uptake in different cellular models, this is the first time it has been specifically employed for a parasite-killing indication. It is also the first time that LND activity has been tested in L. infantum and P. falciparum in vitro models, and in the case of 3BP, additionally in a T. cruzi in vitro model. Although biguanides antiplasmodial properties are widely known, this is the first study where MET activity has been assayed in a L. infantum and T. brucei in vitro model.
Our results show that bioenergetic modulators have greater efficacy on those parasites that are more dependent on glycolysis, as is the case for T. brucei and P. falciparum (Michels et al., 2006; Schalkwyk et al., 2008; Salcedo-Sora et al., 2014). Apart from the specific efficacy of the compounds in the inhibition of target enzymes, such differential parasiticidal activity profile of the studied compounds may be due to the contrasting life-condition adaptations and physiological particularities of each organism. Thus, whereas T. cruzi and Plasmodium spp. are hosted in the cytoplasm of the mammalian host cell during their asexual intracellular stage, and T. brucei prefers multiplying in the host bloodstream thereby exploiting its glucose serum levels, Leishmania spp. show special metabolic features as a result of their adaptation to the low glucose concentration in PV (Naderer and McConville, 2008). Although glucose oxidation plays an important role in Leishmania spp. (McConville et al., 2015), these parasites were less susceptible to glycolysis inhibitors than other assayed parasites. The apparent lack of activity of these compounds may be due to their low access to the parasitophorus vacuole where Leishmania amastigotes reside.
DCA is the only bioenergetic modulator we have found to have antiparasitic activity at the asexual replicative stages within the host of the three genera of parasites studied. It reduced the parasite burden of infected cells in trypanosomatids and the number of infected cells for L. infantum in a dose-dependent manner. The main target of DCA is pyruvate dehydrogenase kinase (PDK) (Stacpoole et al., 1998). PDK inhibits the activity of pyruvate dehydrogenase complex (PDH) which regulates the entrance of pyruvate —the end-product of glycolysis— to the mitochondria and its transformation to acetyl-CoA to start the citric acid cycle. Thus, when DCA inhibits PDK it is redirecting pyruvate to mitochondria and restoring mitochondrial respiration. Genes encoding for PDK have been found in T. brucei, T. cruzi, and L. major (Parsons et al., 2005). However, bloodstream T. brucei is known to have an alternative mitochondrial oxidase non-dependent on pyruvate metabolism, and therefore, PDK is not involved (Chaudhuri et al., 1998). This fact suggests there must be other possible targets of DCA still unknown in this stage of T. brucei. The high IC50 observed for DCA in T. cruzi may suggest that this is not an essential target at this stage, however low accessibility of the compound to the parasite enzyme and or its specificity may also be a cause for its reduced efficacy. No PDK genetic sequences have been identified in P. falciparum (Ward et al., 2004). However, our results show a dose-dependent inhibition of parasite growth by DCA on the intra-erythrocytic stage of P. falciparum, suggesting additional effects of this compound on other essential steps for parasite survival in a non-host cell dependent manner due to the basic metabolism of erythrocytes. The ability of this compound to restore mitochondrial activity may also lead to a decrease of pyruvate availability for other biosynthetic pathways, such as alanine biosynthesis (Tielens and van Hellemond, 2009). The parasite-killing activity of this drug could, therefore, be enhanced by a lack of key proteins for parasite survival. Whilst several studies have shown the efficacy of DCA in rapidly reducing the concentration of lactic acidosis, a common complication of severe malaria strongly related to death in children (Agbenyega et al., 2003), this is the first time that its antiplasmodial activity has been demonstrated.
In addition, DCA activity may also be boosted through actions triggered in the host cell. Many protozoans such as Leishmania and Toxoplasma activate and stabilize HIF1A in the host cell (Wiley et al., 2010; Singh et al., 2012). HIF1A controls the cellular response to low oxygen stress inducing the transcription of genes related to the metabolic switch from oxidative phosphorylation to glycolytic profile, angiogenesis, and apoptosis inhibition (Semenza, 2012). Such a strategy may help these parasites to survive by taking advantage of the increase in macromolecules provided by glycolytic metabolism and the induced inhibition of host cell apoptosis. It has been recently described that the DCA anti-tumor effect also takes place by decreasing HIF1A activity in cancer cells (Sutendra et al., 2013). As a consequence, the observed DCA parasite burden reduction may be related to a decreased HIF1A activity and, in turn, the loss of the metabolic advantages that the host cell provides to its undesirable guests.
We found that the glycolysis inhibitor 3BP showed antiparasitic activity on T. brucei and L. infantum intracellular amastigotes, with potential for VL and African trypanosomiasis treatment. Previous studies have reported some activity on T. brucei, inhibiting motility and growth at 13.4 μM (Barnard et al., 1993), which corroborates our results. Nevertheless, we found no efficacy of 3BP against either T. cruzi or P. falciparum asexual replicative forms despite these parasites being also highly dependent on glycolysis. The main target of 3BP is GAPDH, an enzyme of the glycolytic pathway which, in the case of kinetoplastida, is localized mainly in the glycosomes. Some Leishmania species also have a cytosolic GAPDH isoenzyme shown to play a significant role in VL (Zhang et al., 2013). The efficacy we observed on Leishmania may therefore be due to the inhibition of both isoenzymes. A predictive model led to the conclusion that slight depletions of glycosomal GAPDH result in complete impairment of growth and ATP synthesis in L. infantum amastigotes (Subramanian et al., 2015), thus reinforcing the role of this enzyme for parasite-killing purposes. Furthermore, an increase in GAPDH expression in Leishmania spp. has been related to a resistance to NO (Rios et al., 2015), a basic molecule for intracellular parasite clearance (Mauël et al., 1991). Blocking GAPDH with 3BP would avoid this resistance and allow parasite-killing. Despite 3BP apparently being safe in rodents, where only a reduction in body weight was recorded (Schaefer et al., 2012), 3BP could not be a therapeutic option because of its toxicity in humans (Barnard et al., 1993). However, its molecular target can still be considered for further research.
2DG has been used as a substrate to measure glucose intake in many cell systems due to its condition as a mannose analog that cannot be further metabolized, and also in PET scanning for cancer diagnostic purposes. It blocks glycolysis because it cannot be further metabolized after phosphorylation by the first enzyme of the glycolysis pathway, hexokinase (HK) (Nirenberg and Hogg, 1958). HK isotypes are overexpressed in cancer cells and bound to the mitochondria, thus contributing to the maintenance of the Warburg effect (Bustamante and Pedersen, 1977). LND inhibits HK1 mitochondrial-bounding in tumor cells (Paggi et al., 1988), and has been reported to also inhibit that of T. brucei (Chambers et al., 2008). The efficacy of LND on trypanosomatids has been described and tested on several species, including T. cruzi epimastigotes (Turrens, 1986), T. brucei bloodstream and procyclic forms (Chambers et al., 2008) and L. mexicana promastigotes (Turrens and Cazzulo, 1987). We found an IC50 for T. cruzi amastigotes very close to that previously observed in epimastigotes (IC50 = 80 μM) (Turrens, 1986) and even lower for BS forms of T.brucei in comparison to former authors (IC50 = 50 μM) (Chambers et al., 2008). In contrast, no effect was detected on L. infantum promastigotes. Results previously presented by other authors demonstrate that only a leishmaniostatic effect was observed for L. mexicana promastigotes at concentrations as high as 0.5 mM (Turrens and Cazzulo, 1987). Both 2DG and LND showed antiplasmodial activity on P. falciparum. 2DG has been previously reported to interfere with glucose uptake in P. falciparum, with parasite glycolysis, and with the synthesis of glycosylated macromolecules that play a key role in the intraerythrocytic stage development of trophozoites, therefore reducing their viability (Naik et al., 2000; Schalkwyk et al., 2008). This is the first time LND has been used as an antiplasmodial compound. Because it resulted in 50% reduction of P. falciparum viability at concentrations of 209.13 μM, it emerges as a potential candidate for future malaria treatment.
Whilst biguanides were initially developed as a potential treatments against malaria, MET's ability to decrease blood sugar levels and the discovery of more powerful candidates to fight plasmodium, has resulted in its employment for type II diabetes control. In this study, we have confirmed the previously described MET antiplasmodial activity (Sweeney et al., 2003) and report, for the first time, its efficacy against T. cruzi and T. brucei. MET is thought to block the mitochondrial respiratory-chain complex I. The presence of mitochondrial complex I in Trypanosomatida has been predicted, and all of its components identified, but electron transfer through complex I in T. brucei BSF has been demonstrated not essential for this parasite stage viability (Surve et al., 2012). Thus, the mechanism why MET reduced T. brucei survival still remains unknown. Complex I inhibition by MET leads to metabolic and energetic stress, and activation of AMPK, thus causing down regulation of ATP-consuming processes and pushing the cell into a quiescent state (Fryer et al., 2002). However, if cells lack the ability to cope with energetic stress, as may be the case for tumor cells and highly glycolytic parasites, a metabolic crisis develops leading to death. In addition, the inhibition of the mitochondrial respiratory-chain complex I by MET in host cells reduces O2 consumption as the final acceptor of electrons in the electron transport chain. The subsequent redistribution of intracellular O2 promotes hydroxylation of HIF1A by procollagen-proline dioxygenase (P4HA) and its destruction in the proteasome (Takiyama et al., 2011). As described above, HIF1A appears to be a key component for parasite survival, perhaps opening new avenues for anti-parasite therapeutic intervention. In the case of P. falciparum, there is an alternative NADH dehydrogenase in substitution of the classic complex I (Biagini et al., 2006). P. falciparum relies on its respiratory chain to support pyrimidine biosynthesis, and to maintain the essential mitochondrial membrane potential. Antiplasmodial activity of MET is explained by the disruption on metabolic pathways related to folates, responsible for purine and pyrimidine synthesis, for catabolism of several amino acids, and for glutathione synthesis, thus hindering parasite survival (Bridges et al., 2014).
The mTOR route is a highly conserved pathway in eukaryotic organisms, that regulates the balance between catabolism and anabolism according to cellular needs by acting on ATP-generating routes, DNA transcription, synthesis of biological molecules, autophagy, and cell death (Sabatini and Laplante, 2012). In trypanosomatids, TORC2 orthologue is the only TOR complex susceptible to SIR inhibition. TORC2 is involved in cytoskeletal actin reorganization, playing a key role during host cell invasion and parasite survival (Barquilla et al., 2008). In our model, low concentrations of SIR were needed to induce T. brucei parasite depletion. The sensitivity of this parasite stage to SIR was already known as a previous study pointed to an even lower IC50 value than the one we obtained (IC50 = 152 nM) (Barquilla et al., 2008). However, this may be reflecting the different sensitivity of the different T. brucei strains used. Curiously enough, SIR did not affect the viability of the intracellular forms of the other trypanosomatids analysed.In contrast, an increase of parasite load was observed in the case of Leishmania. Similar results have been reported in other intravesicular parasites, such as different Leishmania species (Schaible et al., 1999; Pinheiro et al., 2009; Jaramillo et al., 2011) or even in P. berghei during its intrahepatic stage when the parasite inhabits non-acidified PV (Hanson et al., 2013). It has been suggested that mTOR inhibition by SIR triggers autophagy and other processes in the host cell, providing them with nutrients for their survival and parasite replication (Brunton et al., 2013). Such processes in the host cell may provide a feasible explanation since autophagy has been reported to promote the generation and maturation of replicative vacuoles of other intracellular pathogens, such as Coxiella sp. (Gutierrez et al., 2005). Moreover, the mTOR route is related to the production of NO in innate immune cells (Weichhart et al., 2008), thus impairing intracellular parasite clearance by blocking mTOR activity when SIR is administered.
In case of the intraerythrocytic stages of P. falciparum, our results concur with previous studies, obtaining an IC50 around 2,5 μM (Bell et al., 1994; Yap et al., 2014). Erythrocytes have high avidity for SIR, therefore most of it reaching the bloodstream is sequestered in RBCs and bound to their immunophilin binding protein FKBP12 (Trepanier et al., 1998). RBCs have an iron dependent mTOR pathway that regulates hemoglobin production and erythrogenesis. However, SIR has shown only modest effect on RBCs in contrast to other mTOR inhibitors responsible for anemia and the decrease in different blood cell populations (Knight et al., 2014). Besides, whilst some authors have even indicated the expression of mTORC1 and C2 proteins in the proteome of mature erythrocytes, there is no evidence of functional proteins (Alessandro et al., 2010). It may be assumed, therefore, that erythrocytes concentrate SIR, exposing the intracellular P. falciparum forms to high concentrations, and that the observed growth inhibitory effect relies purely on the parasite-drug interaction. To date no Plasmodium orthologues of mTOR have been found (van Dam et al., 2011). Nevertheless, SIR has been reported as developing its action by inhibiting the activity of the only FKBP described in P. falciparum (PfFKBP35), which is involved in protein folding and stabilizing, and is an essential chaperone, thus affecting parasite viability (Monaghan and Bell, 2005).
All the concentrations used in this project were selected for being non-cytotoxic to the in vitro models of host cells for each parasite, which provides limited information since toxicity to other cell types or tissues cannot be discarded. However, some of these compounds have a long track record of in vitro and in vivo testing for other purposes or are even commercially available —as it is the case of MET, SIR—. Hence, there is a plethora of information about safety and adverse events of these compounds to facilitate the repositioning process.
In conclusion, the activity of several compounds used in anti-cancer research were studied in different protozoan parasites in vitro models based in the principle that highly metabolically active and proliferation committed cells present an increased glycolytic metabolism and glucose uptake. The results show that metabolic pathways related to ATP and biomass production are promising targets, and drugs with proven effect on these routes are potential candidates for repurposing. This broad principle might offer an economic way to prevent a wide range of neglected parasitic diseases by simply repurposing of existing commercially-approved drugs or in advance stage of development to this new indication. However, more research has to be carried out as the underlying mechanisms of action of the assayed compounds on each parasite metabolism have to be further characterized.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
L. infantum JPC strain (MCAN/ES/1998/LLM-724) was kindly provided by Dr. Javier Moreno and Dr. Eugenia Carrillo, ISCIII Madrid, Spain. This work was supported by the Spanish Ministry of Economy and Competitiveness grant BES-2011-046954.
Contributor Information
Alhelí Rodríguez-Cortés, Email: Alheli.Rodriguez@uab.cat.
Jordi Alberola, Email: Jordi.Alberola@uab.cat.
References
- Agbenyega T., Planche T., Bedu-Addo G., Ansong D., Owusu-Ofori A., Bhattaram V.A., Nagaraja N.V., Shroads A.L., Henderson G.N., Hutson A.D., Derendorf H., Krishna S., Stacpoole P.W. Population kinetics, efficacy, and safety of dichloroacetate for lactic acidosis due to severe malaria in children. J. Clin. Pharmacol. 2003;43:386–396. doi: 10.1177/0091270003251392. [DOI] [PubMed] [Google Scholar]
- Alessandro A.D., Righetti P.G., Zolla L. The red blood cell proteome and interactome: an update. J. Proteome Res. 2010;9:144–163. doi: 10.1021/pr900831f. [DOI] [PubMed] [Google Scholar]
- Barnard J.P., Reynafarje B., Pedersens L. Glucose catabolism in African Trypanosomes. Evidence that the terminal step is catalyzed by a pyruvate transporter capable of facilitating uptake of toxic analogs. J. Biol. Chem. 1993;268:3654–3661. [PubMed] [Google Scholar]
- Barquilla A., Crespo J.L., Navarro M. Rapamycin inhibits trypanosome cell growth by preventing TOR complex 2 formation. Proc. Natl. Acad. Sci. U.S.A. 2008;105:14579–14584. doi: 10.1073/pnas.0802668105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A., Wernli B., Franklin R.M. Roles of peptidyl-prolyl CIS-trans isomerase and calcineurin in the mechanisms of antimalarial action of cyclosporin a, FK506, and rapamycin. Biochem. Pharmacol. 1994;48:495–503. doi: 10.1016/0006-2952(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Besteiro S., Biran M., Biteau N., Coustou V., Baltz T., Canioni P., Bringaud F. Succinate secreted by Trypanosoma brucei is produced by a novel and unique glycosomal enzyme, NADH-dependent fumarate reductase. J. Biol. Chem. 2002;277:38001–38012. doi: 10.1074/jbc.M201759200. [DOI] [PubMed] [Google Scholar]
- Biagini G.A., Viriyavejakul P., O ’neill P.M., Bray P.G., Ward S.A. Functional characterization and target validation of alternative Complex I of Plasmodium falciparum mitochondria. Antimicrob. Agents Chemother. 2006;50:1841–1851. doi: 10.1128/AAC.50.5.1841-1851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges H.R., Jones A.J.Y., Pollak M.N., Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 2014;462:475–487. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J., Steele S., Ziehr B., Moorman N., Kawula T. Feeding uninvited guests: mTOR and AMPK set the table for intracellular pathogens. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante E., Pedersen P.L. High aerobic glycolysis of rat hepatoma cells in culture: role of mitochondrial hexokinase. Proc. Natl. Acad. Sci. U.S.A. 1977;74:3735–3739. doi: 10.1073/pnas.74.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavaci A.M.C., Bustamante J.M., Padilla A.M., Brandan C.M.P., Simpson L.J., Xu D., Boehlke C.L., Tarleton R.L. In vitro and in vivo high-throughput assays for the testing of Anti-trypanosoma cruzi compounds. PLoS Neglected Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J.W., Fowler M.L., Morris M.T., Morris J.C. The anti-trypanosomal agent lonidamine inhibits Trypanosoma brucei hexokinase 1. Mol. Biochem. Parasitol. 2008;158:202–207. doi: 10.1016/j.molbiopara.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Chaudhuri M., Ajayi W., Hill G.C. Biochemical and molecular properties of the Trypanosoma brucei alternative oxidase. Mol. Biochem. Parasitol. 1998;95:53–68. doi: 10.1016/s0166-6851(98)00091-7. [DOI] [PubMed] [Google Scholar]
- Coley A.F., Dodson H.C., Morris M.T., Morris J.C. Glycolysis in the african trypanosome: targeting enzymes and their subcellular compartments for therapeutic development. Mol. Biol. Int. 2011;2011:123702. doi: 10.4061/2011/123702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düvel K., Yecies J.L., Menon S., Raman P., Alex I., Souza A.L., Triantafellow E., Ma Q., Gorski R., Cleaver S., Heiden M.G. Vander, Mackeigan J.P., Finan P.M., Clish C.B., Murphy L.O., Manning B.D. Activation of a metabolic gene regulatory network downstream of mtor complex 1. Mol. Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer L.G.D., Parbu-Patel A., Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- Ganapathy-Kanniappan Sh, Geschwind J.F.H., Kunjithapatham R., Buijs M., Vossen J.A., Tchernyshyov I., Cole R.N., Syed L.H., Rao P.P., Ota Sh, Vali M. Glyceraldehyde-3-phosphate Dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell death. Anticancer Res. 2009;29:4909–4918. [PMC free article] [PubMed] [Google Scholar]
- Ganapathy-Kanniappan S., Geschwind J.F.H., Kunjithapatham R., Buijs M., Syed L.H., Rao P.P., Ota S., Vali M. The pyruvic acid analog 3-bromopyruvate interferes with the tetrazolium reagent MTS in the evaluation of cytotoxicity. Assay Drug Dev. Technol. 2010;8:258–262. doi: 10.1089/adt.2009.0226. [DOI] [PubMed] [Google Scholar]
- Granchi C.M., Minutolo F. Anticancer agents that counteract tumor glycolysis. ChemMedChem. 2013;7:1318–1350. doi: 10.1002/cmdc.201200176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M.G., Vázquez C.L., Munafó D.B., Zoppino F.C.M., Berón W., Rabinovitch M., Colombo M.I. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell Microbiol. 2005;7:981–993. doi: 10.1111/j.1462-5822.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- Hanson K.K., Ressurreição A.S., Buchholz K., Prudêncio M., Herman-ornelas J.D. Torins are potent antimalarials that block replenishment of Plasmodium liver stage parasitophorous vacuole membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 2013;110:e2838–e2847. doi: 10.1073/pnas.1306097110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M. A method for determination of hemoglobin in plasma by near-ultraviolet spectrophotometry. Scand. J. Clin. Lab. Invest. 1959;11:66–70. doi: 10.3109/00365515909060410. [DOI] [PubMed] [Google Scholar]
- Hardie D.G. Sensing of energy and nutrients by AMP-activated protein kinase 1 – 4. Am. J. Clin. Nutr. 2011;93:891S–896S. doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- Jacobs R.T., Nare B., Phillips M.A. State of the art in African trypanosome drug discovery. Curr. Top. Med. Chem. 2011;11:1255–1274. doi: 10.2174/156802611795429167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo M., Gomez M.A., Larsson O., Shio M.T., Topisirovic I., Contreras I., Luxenburg R., Rosenfeld A., Colina R., McMaster R.W., Olivier M., Costa-Mattioli M., Sonenberg N. Leishmania repression of host translation through mTOR cleavage is required for parasite survival and infection. Cell Host Microbe. 2011;9:331–341. doi: 10.1016/j.chom.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Knight Z. a, Schmidt S.F., Birsoy K., Tan K., Friedman J.M. A critical role for mTORC1 in erythropoiesis and anemia. Elife. 2014;3 doi: 10.7554/eLife.01913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y.H., Pedersen P.L., Geschwind J.F. Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Canc. Lett. 2001;173:83–91. doi: 10.1016/s0304-3835(01)00667-x. [DOI] [PubMed] [Google Scholar]
- Maia C., Rolão N., Nunes M., Gonçalves L., Campino L. Infectivity of five different types of macrophages by Leishmania infantum. Acta Trop. 2007;103:150–155. doi: 10.1016/j.actatropica.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Mauël J., Ransijn a, Buchmüller-Rouiller Y. Killing of Leishmania parasites in activated murine macrophages is based on an L-arginine-dependent process that produces nitrogen derivatives. J. Leukoc. Biol. 1991;49:73–82. doi: 10.1002/jlb.49.1.73. [DOI] [PubMed] [Google Scholar]
- Maugeri D.A., Cannata J.J.B., Cazzulo J. Glucose metabolism in Trypanosoma cruzi. Essays Biochem. 2011;51:15–30. doi: 10.1042/bse0510015. [DOI] [PubMed] [Google Scholar]
- Maugeri D.A., Cazzulo J.J., Burchmore R.J.S., Barrett M.P., Ogbunude P.O.J. Pentose phosphate metabolism in Leishmania mexicana. Mol. Biochem. Parasitol. 2003;130:117–125. doi: 10.1016/s0166-6851(03)00173-7. [DOI] [PubMed] [Google Scholar]
- McConville M.J., Saunders E.C., Kloehn J., Dagley M.J. Leishmania carbon metabolism in the macrophage phagolysosome-feast or famine? F1000Research. 2015;4:938. doi: 10.12688/f1000research.6724.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelakis E.D., Webster L., Mackey J.R. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br. J. Canc. 2008;99:989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels P. a M., Bringaud F., Herman M., Hannaert V. Metabolic functions of glycosomes in trypanosomatids. Biochim. Biophys. Acta. 2006;1763:1463–1477. doi: 10.1016/j.bbamcr.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Mita M.M., Mita A., Rowinsky E.K. The molecular target of rapamycin (mTOR) as a therapeutic target against cancer. Canc. Biol. Ther. 2003;2:S169–S177. [PubMed] [Google Scholar]
- Monaghan P., Bell A. A Plasmodium falciparum FK506-binding protein (FKBP) with peptidyl-prolyl cis-trans isomerase and chaperone activities. Mol. Biochem. Parasitol. 2005;139:185–195. doi: 10.1016/j.molbiopara.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Naderer T., McConville M.J. The Leishmania-macrophage interaction: a metabolic perspective. Cell Microbiol. 2008;10:301–308. doi: 10.1111/j.1462-5822.2007.01096.x. [DOI] [PubMed] [Google Scholar]
- Naik R.S., Davidson E. a, Gowda D.C. Developmental stage-specific biosynthesis of glycosylphosphatidylinositol anchors in intraerythrocytic Plasmodium falciparum and its inhibition in a novel manner by mannosamine. J. Biol. Chem. 2000;275:24506–24511. doi: 10.1074/jbc.M002151200. [DOI] [PubMed] [Google Scholar]
- Nirenberg M.W., Hogg J.F. Inhibition of anaerobic glycolysis in Ehrlich ascites tumor cells by 2-deoxy-D-glucose. Canc. Res. 1958;18:518–521. [PubMed] [Google Scholar]
- Nwaka S., Ramirez B., Brun R., Maes L., Douglas F., Ridley R. Advancing drug innovation for neglected diseases-criteria for lead progression. PLoS Neglected Trop. Dis. 2009;3:e440. doi: 10.1371/journal.pntd.0000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M.R., Doran E., Halestrap A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000;348:607–614. [PMC free article] [PubMed] [Google Scholar]
- Paggi M.G., Fanciulli M., Perrotti N., Floridi A., Zeuli M., Silvestrini B., Caputo A. The role of mitochondrial hexokinase in neoplastic phenotype and its sensitivity to lonidamine. Ann. N. Y. Acad. Sci. 1988;551:358–360. doi: 10.1111/j.1749-6632.1988.tb22362.x. [DOI] [PubMed] [Google Scholar]
- Parsons M., Worthey E. a, Ward P.N., Mottram J.C. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genom. 2005;6:127. doi: 10.1186/1471-2164-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkler G., du Toit F., Adams W., Rautenbach M., Palm D.C., van Niekerk D.D., Snoep J.L. Construction and validation of a detailed kinetic model of glycolysis in Plasmodium falciparum. FEBS J. 2015;282:1481–1511. doi: 10.1111/febs.13237. [DOI] [PubMed] [Google Scholar]
- Pinheiro R.O., Nunes M.P., Pinheiro C.S., D'Avila H., Bozza P.T., Takiya C.M., Côrte-Real S., Freire-de-Lima C.G., DosReis G. a. Induction of autophagy correlates with increased parasite load of Leishmania amazonensis in BALB/c but not C57BL/6 macrophages. Microb. Infect. 2009;11:181–190. doi: 10.1016/j.micinf.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Renslo A.R., McKerrow J.H. Drug discovery and development for neglected parasitic diseases. Nat. Chem. Biol. 2006;2:701–710. doi: 10.1038/nchembio837. [DOI] [PubMed] [Google Scholar]
- Rios M.C., Silva W.R.T., Azevedo A.F., Santos P.L., Teixeira S.A., Muscará M.N., Thomazzi S.M., Almeida R.P., Fernandes R.P.M., Scher R. Expression of glyceraldehyde 3-phosphate dehydrogenase is enhanced in Leishmania spp naturally resistant to nitric oxide. Genet. Mol. Res. 2015;14:7113–7121. doi: 10.4238/2015.June.29.4. [DOI] [PubMed] [Google Scholar]
- Sabatini D.M., Laplante M. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo-Sora E., Caamano-Gutierrez E., Ward S.A., Biagini G.A. The proliferating cell hypothesis: a metabolic framework for Plasmodium growth and development.pdf. Trends Parasitol. 2014;30:170–175. doi: 10.1016/j.pt.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldivia M., Barquilla A., Bart J.-M., Diaz-González R., Hall M.N., Navarro M. Target of rapamycin (TOR) kinase in Trypanosoma brucei: an extended family. Biochem. Soc. Trans. 2013;41:934–938. doi: 10.1042/BST20130052. [DOI] [PubMed] [Google Scholar]
- Scatena R., Bottoni P., Pontoglio A., Mastrototaro L., Giardina B. Glycolytic enzyme inhibitors in cancer treatment. Expet Opin. Invest. Drugs. 2008;17:1533–1545. doi: 10.1517/13543784.17.10.1533. [DOI] [PubMed] [Google Scholar]
- Schaefer N.G., Geschwind J.F., Engles J., Buchanan J.W., Wahl R.L. Systemic administration of 3-bromopyruvate in treating disseminated aggressive lymphoma. Transl. Res. 2012;159:51–57. doi: 10.1016/j.trsl.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Schaible U.E., Schlesinger P.H., Steinberg T.H., Mangel W.F., Kobayashi T., Russell D.G. Parasitophorous vacuoles of Leishmania mexicana acquire macromolecules from the host cell cytosol via two independent routes. J. Cell Sci. 1999;112:681–693. doi: 10.1242/jcs.112.5.681. [DOI] [PubMed] [Google Scholar]
- Schalkwyk D.A. Van, Priebe W., Saliba K.J. The inhibitory effect of 2-halo derivatives of D -glucose on glycolysis and on the proliferation of the human malaria parasite Plasmodium falciparum. J. Pharmacol. Exp. Therapeut. 2008;327:511–517. doi: 10.1124/jpet.108.141929. [DOI] [PubMed] [Google Scholar]
- Semenza G.L. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah-Simpson S., Lentini G., Dumoulin P.C., Burleigh B.A. Modulation of host central carbon metabolism and in situ glucose uptake by intracellular Trypanosoma cruzi amastigotes. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman I.W. vol. 4. ASM Press; 1998. pp. 49–61. (Malaria: Parasite Biology, Pathogenesis, and Protection). [Google Scholar]
- Singh A.K., Mukhopadhyay C., Biswas S., Singh V.K., Mukhopadhyay C.K. Intracellular pathogen Leishmania donovani activates Hypoxia Inducible Factor-1 by dual mechanism for survival advantage within macrophage. PloS One. 2012;7 doi: 10.1371/journal.pone.0038489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole P., Henderson G., Yan Z., Cornett R., James M. Pharmacokinetics, metabolism, and toxicology of dichloroacetate. Drug Metab. Rev. 1998;30:499–539. doi: 10.3109/03602539808996323. [DOI] [PubMed] [Google Scholar]
- Subramanian A., Jhawar J., Sarkar R.R. Dissecting Leishmania infantum energy metabolism - a systems perspective. PloS One. 2015;10:1–34. doi: 10.1371/journal.pone.0137976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surve S., Heestand M., Panicucci B., Schnaufer A., Parsons M. Enigmatic presence of mitochondrial Complex I in Trypanosoma brucei bloodstream forms. Eukaryot. Cell. 2012;11:183–193. doi: 10.1128/EC.05282-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutendra G., Dromparis P., Kinnaird A., Stenson T.H., Haromy A., Parker J.M.R., McMurtry M.S., Michelakis E.D. Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer. Oncogene. 2013;32:1638–1650. doi: 10.1038/onc.2012.198. [DOI] [PubMed] [Google Scholar]
- Sweeney D., Raymer M.L., Lockwood T.D. Antidiabetic and antimalarial biguanide drugs are metal-interactive antiproteolytic agents. Biochem. Pharmacol. 2003;66:663–677. doi: 10.1016/s0006-2952(03)00338-1. [DOI] [PubMed] [Google Scholar]
- Takiyama Y., Harumi T., Watanabe J., Fujita Y., Honjo J., Shimizu N., Makino Y., Haneda M. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1A expression and oxygen metabolism. Diabetes. 2011;60:981–992. doi: 10.2337/db10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielens A.G.M., van Hellemond J.J. Surprising variety in energy metabolism within Trypanosomatidae. Trends Parasitol. 2009;25:482–490. doi: 10.1016/j.pt.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. Human malaria parasites in continuous culture. Science. 1976;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trepanier D.J., Gallant H., Legatt D.F., Yatscoff R.W. Rapamycin : distribution , pharmacokinetics and therapeutic range investigations : an update. Clin. Biochem. 1998;31:345–351. doi: 10.1016/s0009-9120(98)00048-4. [DOI] [PubMed] [Google Scholar]
- Turrens J.F. Inhibitory action of the antitumor agent lonidamine on mitochondrial respiration of Trypanosoma cruzi and T. brucei. Mol. Biochem. Parasitol. 1986;20:237–241. doi: 10.1016/0166-6851(86)90104-0. [DOI] [PubMed] [Google Scholar]
- Turrens J.F., Cazzulo J.J. Inhibition of growth and respiration of Leishmania mexicana by the antitumor agent lonidamine. Comp. Biochem. Physiol., C. 1987;88:193–196. doi: 10.1016/0742-8413(87)90067-3. [DOI] [PubMed] [Google Scholar]
- van Dam T.J.P., Zwartkruis F.J.T., Bos J.L., Snel B. Evolution of the TOR pathway. J. Mol. Evol. 2011;73:209–220. doi: 10.1007/s00239-011-9469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlinde C.L.M., Hannaert V., Blonski C., Willson M., Périé J.J., Fothergill-Gilmore L.A., Opperdoes F.R., Gelb M.H., Hol W.G.J., Michels P.A.M. Glycolysis as a target for the design of new anti-trypanosome drugs. Drug Resist. Updates. 2001;4:50–65. doi: 10.1054/drup.2000.0177. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Warburg O., Posener K., Negelein E. Ueber den stoffwechsel der tumoren. Biochem. Z. 1924;152:319–344. [Google Scholar]
- Ward P., Equinet L., Packer J., Doerig C. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genom. 2004;5:79. doi: 10.1186/1471-2164-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichhart T., Costantino G., Poglitsch M., Rosner M., Zeyda M., Stuhlmeier K.M., Kolbe T., Stulnig T.M., Hörl W.H., Hengstschläger M., Müller M., Säemann M.D. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Wiley M., Sweeney K.R., Chan D.A., Brown K.M., McMurtrey C., Howard E.W., Giaccia A.J., Blader I.J. Toxoplasma gondii activates Hypoxia-inducible Factor (HIF) by stabilizing the HIF-1 subunit via type I activin-like receptor kinase receptor signaling. J. Biol. Chem. 2010;285:26852–26860. doi: 10.1074/jbc.M110.147041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization WHO | World health Organization. 2020. https://www.who.int/ URL. accessed 2.12.20.
- Yang T.T., Sinai P., Kain S.R. An acid phosphatase assay for quantifying the growth of adherent and nonadherent cells. Anal. Biochem. 1996;241:103–108. doi: 10.1006/abio.1996.0383. [DOI] [PubMed] [Google Scholar]
- Yap A., Azevedo M.F., Gilson P.R., Weiss G.E., O'Neill M.T., Wilson D.W., Crabb B.S., Cowman A.F. Conditional expression of apical membrane antigen 1 in Plasmodium falciparum shows it is required for erythrocyte invasion by merozoites. Cell Microbiol. 2014;16:642–656. doi: 10.1111/cmi.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.W., McCall L.I., Matlashewski G. Role of cytosolic glyceraldehyde-3-phosphate dehydrogenase in visceral organ infection by Leishmania donovani. Eukaryot. Cell. 2013;12:70–77. doi: 10.1128/EC.00263-12. [DOI] [PMC free article] [PubMed] [Google Scholar]