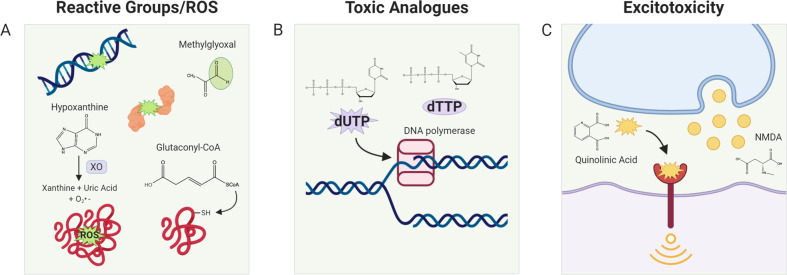
Fig. 2. Different categories of toxic metabolites based on their toxicity mechanism.
a ROS/reactive groups. Some metabolites contain reactive groups such as aldehydes which allow them to form adducts with macromolecules such as DNA and proteins. Others can form reactive oxygen species and similarly damage DNA and proteins through oxidation. The representative ROS forming/reactive metabolites are depicted in the figure. Hypoxanthine is a ROS former, as it produces ROS as it is catabolized. Methylglyoxal is a reactive aldehyde. Glutaconyl-CoA is a reactive metabolite that preferentially reacts with thiol groups. b Toxic analogs. Due to the promiscuity of metabolic enzymes and structural similarity of small metabolites, several metabolites confer their toxicity to cells via misincorporation of wrong nucleotides or competitive inhibition of the enzyme reactions. High dUTP/dTTP ratio promotes misincorporation of dUTP, which leads to DNA damage and eventually cell death c excitotoxicity. Several metabolites can act as a ligand to neurotransmitters and induce hyperactivation of receptor-mediated signaling, leading to cell death. Chemical structures of metabolites were drawn with ChemDraw software.